Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
International Microbiology
versión impresa ISSN 1139-6709
INT. MICROBIOL. vol.7 no.1 mar. 2004
| RESEARCH ARTICLE | |||
|
| |||
| Laboratory diagnosis of
Summary. Sera obtained from 62 patients from four mountain counties in Catalonia (Northeastern Spain), in whom brucellosis had been diagnosed on the basis of clinical evidence and/or personal history, were analyzed using the rose Bengal test, standard serum agglutination test (SAT), Coombs' test, ELISA, and complement fixation. The diagnosis was further confirmed through blood cultures. Clinical evidence, epidemiology, and the results from serologic tests were used to assign patients to one of two groups: group 1 (n = 38) patients had primary infections, whereas group 2 (n = 24) patients had been previously exposed to the microorganism, i.e. re-infection of group 2 individuals occurred after long periods of time during which no active infection by Brucella had been detected. Receiving-operating charts (ROC) were used to determine the diagnostic value of the different tests and to establish discriminant values. Blood culture was a valuable diagnostic tool in group 1 (0.92 sensitivity) but was inappropriate in group 2 (0.08). The combination of positive rose Bengal test and agglutination ≥1/160 was valid for diagnosis in group 1. In group 2, agglutination <1/160 (including negative agglutination) did not rule out brucellosis. The combination of positive rose Bengal test and Coombs' test ≥1/320 was the best diagnostic criterion (0.8 specificity; 1 sensitivity). ELISA (for IgG, IgM, or both) did not improve diagnostic accuracy. [Int Microbiol 2004; 7(1): 53 - 58] Key words: Brucella · human brucellosis · serology · diagnostic criteria | ||
| |||
| Diagnóstico de laboratorio de brucelosis en un área rural endémica en el noreste de España Resumen. A partir del suero de 62 pacientes de tres comarcas de montaña de Cataluña (noreste de España) con brucelosis según los síntomas clínicos y/o historia personal, se probó el valor diagnóstico de diferentes pruebas tales como el test del rosa de Bengala, el test de aglutinación estándar del suero (SAT), el test de Coombs, el test ELISA y el test de fijación del complemento. Para el diagnóstico se realizaron también cultivos de sangre. Basándose en los síntomas clínicos, los datos epidemiológicos y los resultados de las pruebas serológicas, los pacientes se clasificaron en dos grupos: grupo 1 (38 casos), infectados por primera vez, y grupo 2 (24 casos), cuyos pacientes habían padecido una exposición previa al microorganismo, esto es, individuos reinfectados por Brucella después de un largo período sin infección activa. Para determinar el valor diagnóstico de las diferentes pruebas y establecer valores discriminatorios se utilizó el gráfico "receiving-operating chart" (ROC). El cultivo de sangre fue apropiado para el grupo 1 (sensibilidad 0,92), pero no para el grupo 2 (sensibilidad 0,08). La combinación de los test del rosa de Bengala y de aglutinación (≥1/160), tuvieron valor diagnóstico para el grupo 1. Sin embargo, para el grupo 2 el test de aglutinación (<1/160, se incluyen las aglutinaciones negativas) no fue apropiado para la detección de Brucella. El mejor criterio de diagnóstico de brucelosis fue la combinación del test rosa de Bengala y el test de Coombs (≥1/320; especificidad 0,8 y sensibilidad 1). El test ELISA (con IgG o con IgM, o con ambas) no mejoró el diagnóstico de la enfermedad. [Int Microbiol 2004; 7(1):53- 58] Palabras clave: Brucella · brucelosis humana · serología · criterios diagnósticos | Diagnóstico laboratorial de brucelose numa área endêmica no noreste da Espanha Resumo. A partir do soro de 62 pacientes, provenientes de três zonas montanhosas da Cataluña (noreste da Espanha), nos quais havia sido diagnosticado brucelose com base nos sintomas clínicos e/ou uma história pessoal, foi testado o valor diagnóstico de diferentes provas tais como: o teste de rosa de bengala, o teste de aglutinação padrão do soro (SAT), teste de Coombs, o teste ELISA e o teste de fixação do complemento. Para o diagnóstico foram realizados também cultivos de sangue. Com base nos sintomas clínicos, dados epidemiológicos e os resultados do teste sorológico, os pacientes foram classificados em dois grupos: grupo 1 (38 casos), infectados pela primeira vez e o grupo 2 (24 casos), pacientes que haviam sido expostos previamente ao microrganismo, isto é, indivíduos reinfectados por Brucella depois de uma grande período sem infecção ativa. Para se determinar o valor diagnóstico das diferentes provas e estabelecer valores discriminatórios foi utilizado o gráfico "receiving-operating chart" (ROC). Os cultivos de sangue foram apropriados para o grupo 1 (sensibilidade 0,92), mas não para o grupo 2 (sensibilidade 0,08). A combinação dos testes de rosa de Bengala e de aglutinação (≥1/160), tiveram valor diagnóstico para o grupo 1. Sem dúvida, para o grupo 2, o teste de aglutinação (<1/160, são incluídas as aglutinações negativas) não foi adequado para a detecção de Brucella. O melhor critério de diagnóstico para brucelose foi a combinação do teste rosa de bengala e o teste de Coombs (≥1/320; especificidade 0,8 e sensibilidade 1). O teste ELISA (com IgG ou com IgM, ou com ambas) não melhorou o diagnóstico da enfermidade. [Int Microbiol 2004; 7(1):53- 58] Palavras chave: Brucella · brucelose humana · sorologia · critérios diagnósticos |
Introduction
Unequivocal diagnosis of brucellosis requires isolation of the causal agent. Blood culture is the method of choice, but specimens need to be obtained early, and cultures often need long periods of incubation. In addition, failure to detect the pathogen is a frequent occurrence. Although in the last few years PCR-based laboratory tests have been proposed [4,14,16,20], they cannot be considered a routine diagnostic method yet. These limitations make serology the most useful tool for laboratory diagnosis of Brucella infection. Nonetheless, antibody detection is not always sufficient to indicate the existence of active infection, especially in endemic areas in which equivocal serologic profiles among affected individuals are frequent. In addition, insidious clinical forms of brucellosis are often associated with these serologic profiles, thus introducing a high degree of complexity in laboratory diagnosis such that, in many cases, serologic profiles alone are not clear enough to support a reliable diagnosis.
Several reports dealing with the significance of various laboratory tests in the diagnosis of brucellosis have been published [6,12,24], although investigators frequently disagree regarding the criteria. The present, prospective study deals with the usefulness and significance of blood culture and serology tests in the diagnosis of human brucellosis in an endemic area in which livestock farming is the main occupation. The area includes Pallars Jussà, Pallars Sobirà, Alta Ribagorça, and Vall d'Aran, four counties characterized by low densities of human population and high densities of ruminants, including goats, sheep, and cows. Previous evidence supporting the endemic character of brucellosis in these areas has been reported elsewhere [21,22]. The main purpose of this work was to evaluate the roles of classical serological methods and ELISA in the diagnosis of human brucellosis in the populations studied, in order to design diagnostic protocols applicable in rural hospitals.
Materials and methods
Patiens. A total of 62 patients suffering from brucellosis from 1995 to 1998 were studied. All of them were from the above-mentioned counties in Lleida, Spain, a rural area where brucellosis is endemic. Isolation of Brucella, clinical evidence and agglutination test values ≥1/160, or clinical evidence and a Coombs' test with a fourfold increase in agglutination were used as positive diagnostic criteria.
Patients were classified into two groups. Group 1 consisted of 38 patients with primary infection (no personal history of brucellosis) and showing acute clinical symptoms. Group 2 was relatively poorly defined and consisted of 24 patients with evidence of previous infection by Brucella: (i) brucellosis diagnosed previously, or (ii) epidemiological data compatible with long exposure (such as in farmers and veterinarians) to the pathogen and an immune response of "secondary type" (IgG predominating on IgM)
When group 1 was studied, a control population of 346 individuals (control 1) living in the same area was examined ("negative-healthy" population). For group 2, the control group consisted of 55 healthy individuals (control 2) in whom brucellosis had previously been diagnosed and subsequently treated more than 2 years before, with no subsequent symptoms of the disease ("cured" population).
Blood cultures were set up before antibiotic treatment. Two samples were taken at 30-min intervals. Culture and presumptive identification were done according to previously described procedures [21]. Isolates were sent to Laboratorio Regional de Brucelosis, Valladolid, Spain, for confirmatory identification.
Serological methods. For serology, blood samples were centrifuged (3000×g for 10 min) and the serum divided into aliquots and stored at - 20ºC until needed. All sera were evaluated using the rose Bengal test, serum agglutination test, Coombs' test, IgM and IgG ELISA, and complement fixation. Rose Bengal test was done according to the method of Morgan et al. [17] using commercial antigen (L1-M1110, Linear Chemicals SL, Spain). Tube agglutination and Coombs' test were prepared using a suspension of Brucella abortus ATCC 11192 and following the methods described elsewhere [7,23]. For the Coombs' test, antigen was centrifuged at 3000×g for 1 h to eliminate soluble antigen, and the pellet (corpuscular antigen) was suspended in saline. ELISA was done by a modification of the method described by Voller et al. [23]. Briefly, microplates of polystyrene (96 wells) were coated with 100 µl LPS-S of Brucella melitensis 16 M (kindly supplied by Prof. I. Moriyón, University of Navarra, Spain) at 2.5 µg/ml, pH 7.2 PBS. Plates were incubated overnight at 4ºC in a humid environment and washed four times with PBS containing 0.02% Tween 20. A volume of 100 µl of serum to be tested (diluted either 1/50 for IgG or 1/20 for IgM in PBS) was added, and plates were incubated at 37ºC for 45 min and then washed as described above. Subsequently, 100 µl of conjugate was added and plates were incubated again at 37ºC for 1 h. Two different conjugates were used: (i) peroxidase conjugate [goat antibodies to human IgM (µ-chain specific), Sigma A-6907] diluted 1/1000 in PBS (ELISA IgM); (ii) peroxidase-conjugated goat antibodies to human IgG (γ-chain-specific) (Sigma A-6029) diluted 1/1000 in PBS (ELISA IgG). After incubation and final washing, 200 µl of a solution containing 0.04% o-phenylenediamine, 0.04% H2O2 in 0.05 M phosphate-citrate buffer (Sigma FAST OPD, Sigma P9187) was added. After 45 min incubation at room temperature, absorbances at 450 nm were measured using a Behring EL 311 Autoreader (Hoechst). The results (in arbitrary units) were calculated as follows: (ODts- ODngs)/(ODps- ODngs)×100, where ODts is the mean OD of the test sample, ODngs is the mean OD of the negative standard serum, and ODps is the mean OD of the positive standard serum. After establishing intra- and interseries inaccuracies in the ELISA tests, the stability of the samples in plates stored at 4ºC was tested.
Complement fixation using a Brucella abortus S-99 suspension (Brucellosis Reference Laboratory, Santa Fe, Granada, Spain) in veronal buffer (pH 7.2) as antigen was carried out in a veterinary laboratory in La Pobla de Segur (Lleida, Spain). The hemolytic system consisted of 3% sensitized-sheep red blood cell suspension (Biomerieux) and 4 hemolytic units of guinea-pig hemolysin (Dade Behring ORLC25). Complement (guinea-pig complement for the complement fixation reaction, Dade Behring ORAY20) was titrated and 2 complement units were used. Serum samples were inactivated at 56ºC for 30 min and diluted in veronal buffer (from 1:2 to 1:128). General procedures were done as described by Alton et al. [1]. The results were expressed in International Complement-Fixation Test Units (ICFTU), established by the European Union based on an International Standard anti-Brucella abortus serum, available from the Institut für Veterinarienmedizin, Berlin, Germany.
Statistics. Qualitative (rose Bengal) as well as semiquantitative (serum agglutination activity, and Coombs' reaction) tests were converted into ordinal numbers to facilitate statistical analysis.
Receiving-operating charts (ROC) were used to evaluate and compare tests results and to select the best decision levels of each test. Sensitivity, specificity, efficiency, positive predictive value, negative predictive value, and area under the curve (AUC) were calculated for each test. ROC were generated for group 1 patients versus general population (control 1); group 2 patients versus control group 2 with brucellosis history; and every patient (either from group 1 and 2) versus general population (control 1) using the software GraphROC 1.5 for Windows (GR-2028).
Results
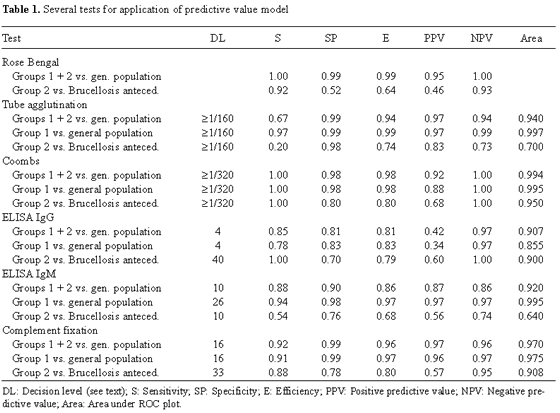
Brucella was isolated from 92.5% of blood cultures from patients belonging to group 1, but only 8.3% of those from patients of group 2. This means that the sensitivity with respect to both groups was 0.66. All patients belonging to group 1 were positive by the rose Bengal test (sensitivity = 1) as were 22 patients of group 2 (sensitivity = 0.92). Three individuals in control group 1 and 26 individuals in control group 2 were also found to be positive using this test. Table 1 summarizes the results obtained when the predictive value model was applied to all studied patients (groups 1 and 2) and to group 2.
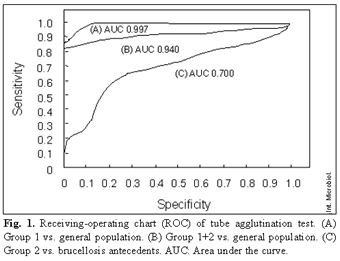
Figure 1 shows the corresponding ROC of the serum agglutination test. The Coombs' test was evaluated considering a discriminant value of 1/320 (maximal discrimination). ELISA (IgG) gave the best results in patients of group 1 and groups 1+2 when value >4 arbitrary units (AU) was considered as the cut-off point. When patients of group 2 were compared with control group 2, a break point ≥40 AU was applied;
Table 1 lists both the specificity and the sensitivity values.
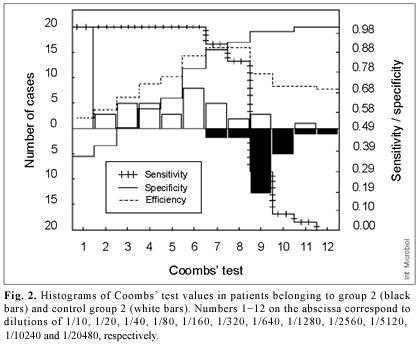
Figure 2 shows differences in the results of the Coombs' test between group 2 and control group 2.
Figure 3 shows the results of ELISA (IgG). The results obtained with the ELISA (IgM) were almost identical to those of the serum agglutination test.
Concerning the complement-fixation results, note that when patients of group 2 were compared with control group 2 (break point >33 ICFTU, Table 1), a sensitivity of 0.88 and a specificity of 0.78 were obtained. Attempts were made to increase sensitivity, but then specificity fell rapidly. Two false negatives were detected in group 1, corresponding to two patients with an immune response in which IgM was predominant.
Discussion
Blood culture results of patients belonging to group 1 demonstrated the high sensitivity of this diagnostic test in primary infected patients. However, the low success of blood cultures from group 2 patients indicated its limitations as a laboratory test in a rural area in which brucellosis is endemic. This conclusion is in agreement with those from other studies in different endemic areas, including Kuwait [18] and Saudi Arabia [13]. The sensitivity level in our study was 0.66, slightly lower than the values reported (0.80) in previous studies [2]. This difference could be a consequence of the high proportion of patients (39%) belonging to group 2.
The low specificity of rose Bengal test in group 2 indicates that, at least in endemic areas, it should not be used as a diagnostic tool, especially in individuals with occupational exposure. However, due to its simplicity, it can be useful in screening [25]. In group 2, there were two false-negatives (sensitivity = 0.92) with the rose Bengal test: two patients professionally exposed suffering from chronic brucellosis with clinical manifestations in osteo-articulations. After other diseases were ruled out, antibiotic treatment succeeded (Coombs' test value >1/1280). Negativity may have been due to the loss of agglutinating capability of the patients' IgG at low pH. Furthermore, antibodies were detected by the Coombs' reaction. Thus, in endemic areas and in patients with a long history of disease, brucellosis cannot be ruled out based on a negative rose Bengal test.
Results of the standard tube agglutination method have to be considered taking into account those reported by other groups. Several studies indicated that an optimal break point of 1/160 gave excellent sensitivity even in endemic regions [3,13,15,19]. However, in other studies [12,24], when clinical evidence suggested brucellosis, even values <1/160 may not rule out the diagnosis. In our study, the sensitivity of the standard tube agglutination method was low (0.67) and only the results in group 1 were similar to those already described. When group 2 was compared with control group 2, sensitivity was 0.2 at a break point of 1/160 and 0.33 at a break point ≥1/80 with specificity of 0.82 and area under the ROC of 0.7. Thus, agglutination tests do not rule out brucellosis in endemic regions such as those in our study.
IgM, agglutinating IgA, and agglutinating IgG are involved in tube serum agglutination [2,12]. Sometimes IgG is masked by blocking antibodies [10] belonging to class IgA. In patients with a long history of brucellosis or in individuals with immunological memory, such as those of group 2, the predominant response is due to IgG, which can be non-agglutinating or blocked; this may explain the low efficiency of the standard tube agglutination test.
Application of Coombs' test, which detects non-agglutinating IgG, improved the accuracy of diagnosis in patients of group 2. When the break point was ≥1/320, the value of sensitivity was 1 in all groups. Specificity, however, was only 0.8 in individuals with a history of brucellosis. This 20% incidence of false-positives is attributable to overlap between antibodies produced during active infection in individuals previously exposed to the microbe and to the presence of residual antibodies in healthy individuals that had suffered from brucellosis previously. Only one healthy individual of the latter group gave a titer >1280. The area of uncertainty ranged from 1/320 to 1/1280 (Fig. 2). Using a break point of 1/640, limitations in the test would be distributed between false-positives (specificity 0.89) and false-negatives (sensitivity 0.91).
Immunoenzymatic tests (ELISA) did not increase the diagnostic yield of classical tests. ELISA IgG gave sensitivities and specificities of 0.85 and 0.81, respectively, in the entire series of patients, whereas values in group 1 were 0.78 and 0.83 (Table 1). In fact, patients belonging to group 1 had a lower IgG response, especially when the disease was diagnosed at its onset. Even in patients of group 2, IgG ELISA did not give better results than obtained with the Coombs' test. With a break point of ≥ 40 AU (maximal sensitivity), the specificity was 0.7. This means that, in fact, the amount of overlap was higher than that detected with the Coombs' test (Fig. 3).
The results of ELISA IgM were identical to those of standard tube agglutination as reported elsewhere [11]. This means that the test was an excellent diagnostic tool for patients of group 1 (IgM producers) but gave poor results with patients of group 2. This confirms that tube agglutination indicates mainly the presence of IgM. In no case did ELISA-IgM improve results obtained with the tube agglutination method. Therefore, the diagnostic values of classical tests for IgM (tube agglutination) and IgG (Coombs' test) were not less than that of ELISA. This conflicts with the results of other authors [5,8,9]. Although classical tests are easy to perform, extreme caution should be exercised in the choice of antigen, experimental procedure, and interpretation of results. Choosing a break points based on a high enough number of individuals is also another variable to be considered.
Due to its ability to detect both IgG and IgM, the complement fixation test was also very useful in diagnosing brucellosis. However, the results did not improve the diagnostic values of tube agglutination and ELISA IgM in group 1 patients, nor those of Coombs' test and ELISA IgG in group 2 patients. These results and the complication of the complement fixation test itself should discourage its use in routine clinical analysis. Moreover, false negative results were obtained in two patients of group 1with positive blood culture and high levels of IgM and IgG, which were undetected. As has already been shown, IgM is much less efficient in complement fixation than IgG. In both patients, a second complement fixation test 15 days later gave accurate results. Perhaps IgM needs a certain period of time after secretion to become active in complement fixation.
Conclusions
Based on our results, the following conclusions can be drawn: (i) Due to the lack of standardized serology tests and differences in human populations, each laboratory should establish its own break point based on the methods used and the population studied. (ii) In the population in this study, laboratory diagnosis in patients with primary infections (group 1) did not cause particular difficulties because blood culture gave excellent sensitivity results, and the combination of rose Bengal (screening test) and tube agglutination ≥1/160 ensured the diagnosis. ELISA IgM can be used although it does not improve the accuracy of diagnosis. (iii) In individuals with previous contact with the microorganism or occupational exposure and symptoms of acute, persistent, and often apparently unspecific infection (very frequent in endemic areas), blood culture gave poor results. In such cases a positive rose Bengal test (screening method) and tube agglutination <1/160 do not rule out brucellosis. A Coombs' test titer of ≥1/320 strongly suggests active infection, although some false positives were detected between 1/320 and 1/1280. In these patients, continuous clinical and analytical control are needed.
Acknowledgements. The generous gift of antigens by Prof. Ignacio Moriyón (University of Navarra, Spain) is gratefully acknowledged. We are indebted to Dr. Manuel Beneria (Veterinary Laboratory of Pobla de Segur) for his help in complement fixation experiments. This work was partially supported by CIRIT (Autonomous Government of Catalonia) grant 1996ACOM00045 and by the Institut Universitari de Salut Pública de Catalunya (1995-1996).
References
1. Alton GG, Jones LM, Angus RD, Verger JM (1998) Techniques for the brucellosis laboratory. Institut National de la Recherche Agronomique, Paris [ Links ]
2. Ariza J, Pellicer T, Pallarés R, Foz A, Gudiol F (1992) Specific antibody profile in human brucellosis. Clin Infect Dis 14:131- 140 [ Links ]
3. Buchanan TM, Sulzer CR, Frix MK, Feldman RA (1974) Brucellosis in the United States, 1960- 1972. An abattoir-associated disease. Part II. Diagnostic aspects. Medicine (Baltimore) 53:415- 425 [ Links ]
4. Cloeckaert A, Verger JM, Grayon M, Grépinet O (1996) Polymorphism at the dnaK locus of Brucella species and identification of a Brucella melitensis species-specific marker. J Med Microbiol 45:200- 205 [ Links ]
5. De Klerk E, Anderson R (1985) Comparative evaluation of the enzyme-linked immunosorbent assay in the laboratory diagnosis of brucellosis. J Clin Microbiol 21:381- 386 [ Links ]
6. Díaz R, Moriyón I (1989) Laboratory techniques in the diagnosis of human brucellosis. In: Young EJ, Corbel MJ (ed) Brucellosis: Clinical and Laboratory Aspects. CRC, Boca Raton, FL, pp. 73- 83 [ Links ]
7. Foz A, Garriga S (1954) Relation entre la fixation du complement et "les anticorps incomplets" (test de Coombs) dans le brucellose humaine. Rev Immunol 18:288- 289 [ Links ]
8. Gad El-Rab MO, Kambal AM (1998) Evaluation of a Brucella enzyme immunoassay test (ELISA) in comparison with bacteriological culture and agglutination. J Infect 36:197- 201 [ Links ]
9. Gazapo E, Gonzalez J, Subiza JL, Baquero M, Gil J, de la Concha EG (1989) Changes in IgM and IgG antibody concentrations in brucellosis over time: Importance for diagnosis and follow-up. J Infect Dis 159:219- 225 [ Links ]
10. Griffitts J (1947) Agglutination and an agglutinin-"blocking" property in serums from known cases of brucellosis. Public Health Rep 62:865- 875 [ Links ]
11. Kerr WR, Coghlan JD, Payne DJ, Robertson L (1966) The laboratory diagnosis of chronic brucellosis. Lancet 2:1181- 1183 [ Links ]
12. Kerr WR, McCaughey WJ, Coghlan JD, Payne DJ, Quaife RA, Robertson L, Farrell ID (1968) Techniques and interpretations in the serological diagnosis of brucellosis in man. J Med Microbiol 1:181- 93 [ Links ]
13. Kiel FW, Yousuf M (1987) Analysis of 506 consecutive positive serologic tests for brucellosis in Saudi Arabia. J Clin Microbiol 25:1384- 1387 [ Links ]
14. Leal-Klevezas DS, Martínez-Vázquez IO, López-Merino A, Martínez-Soriano JP (1995) Single-step PCR for detection of Brucella spp. from blood and milk of infected animals. J Clin Microbiol 33:3087- 3090 [ Links ]
15. Martín S, Guinea L, Carrero P, Visedo R, García S, Calvo T, Reverte D (1992) El diagnóstico de la brucelosis en un área endémica. Valoración de las pruebas diagnósticas habituales. Med Clin 98:481- 485 [ Links ]
16. Matar GM, Khneisser IA, Abdelnoor AM (1996) Rapid laboratory confirmation of human brucellosis by PCR analysis of a target sequence on the 31-kilodalton Brucella antigen DNA. J Clin Microbiol 34:477- 478 [ Links ]
17. Morgan WJ, Mackinnon DJ, Lawson JR, Cullen GA (1969) The rose Bengal plate agglutination test in the diagnosis of brucellosis. Vet Rec 85:636- 641 [ Links ]
18. Mousa AR, Elhag KM, Khogali M, Marafie AA (1988) The nature of human brucellosis in Kuwait: study of 379 cases. Rev Infect Dis 10:211- 217 [ Links ]
19. Rodríguez A (1988) Diagnóstico de la brucelosis humana. Rev Esp Reumatol 10:211- 217 [ Links ]
20. Romero C, Gamazo C, Pardo M, López-Goñi I (1995) Specific detection of Brucella DNA by PCR. J Clin Microbiol 33:615- 617 [ Links ]
21. Serra J, Godoy P (2000) Incidencia, etiología y epidemiología de la brucelosis en un área rural de la provincia de Lleida. Rev Esp Salud Pública 74:45- 53 [ Links ]
22. Serra J, Pujol R, Godoy P (2000) Estudio seroepidemiológico de la brucelosis en un área rural endémica. Enf Infec Microbiol Clin 18:74- 78 [ Links ]
23. Voller A, Bartlett B, Bidwell DE (1978) Enzyme immunoassays with special reference to ELISA techniques. J Clin Pathol 31:507- 520 [ Links ]
24. Young EJ (1991) Serologic diagnosis of human brucellosis: analysis of 214 cases by agglutination tests and review of the literature. Rev Infect Dis 13:359- 372 [ Links ]
25. Zancada F, Roldán A, Férnandez A, Jiménez J, Agulla A (1992) Brucelosis: estudio clinicoserológico en un área de salud rural. Rev Clin Esp 119:8- 12 [ Links ]