Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
International Microbiology
versión impresa ISSN 1139-6709
INT. MICROBIOL. vol.8 no.1 mar. 2005
| RESEARCH REVIEW | |||
|
| |||
| A comparison of ready-to-use systems for evaluating the microbiological quality of acidic fruit juices using non-pasteurized orange juice as an experimental model Summary. Several alternative analytical methods are currently available for the rapid microbiological testing of food. Due to their many advantages, particularly their convenience of use, the popularity of ready-to-use systems for the enumeration of hygiene indicator microorganisms is increasing. However, the ability of these systems to enumerate stressed microorganisms, such as those that may be found growing in acidic foods, is unknown. Therefore, the aim of this study was to evaluate the performance of Petrifilm and SimPlate plates for the enumeration of total aerobes and fungi (yeasts and molds) in acidic fruit juices, using non-pasteurized orange juice as an experimental model. The samples were analyzed before and after neutralization of pH, and the results were compared with those obtained using conventional procedures, i.e. pour-plates containing Standard Methods Agar, acidified potato dextrose agar, or dichloran-glycerol agar. The results obtained with Petrifilm and SimPlate for counts of mesophilic aerobes as well as for yeast and mold correlated well with those obtained using conventional procedures. Although no statistically significant differences were observed between counts of non-neutralized and neutralized samples (α ≤ 0.05), better correlation indexes were observed in the neutralized samples. Both Petrifilm and SimPlate proved to be good alternative methods for testing the microbiological quality of acidic fruit juices. [Int Microbiol 2005; 8(1):49-53] Key words: microbiological analysis · acidic fruit juices · rapid methods · Petrifilm · SimPlate | ||
| |||
| Comparación de algunos sistemas listos para usar en la evaluación de la calidad microbiológica de zumos de frutas ácidas, usando zumo de naranja no pasteurizado como modelo experimental Resumen. Actualmente se dispone de varios métodos analíticos alternativos para el análisis microbiológico rápido de alimentos. Debido a sus muchas ventajas, especialmente su facilidad de uso, cada vez es mayor la popularidad de los sistemas listos para usar en la enumeración de los microorganismos indicadores de las condiciones higiénicas. Sin embargo, no se sabe cómo actúan estos sistemas en la enumeración de microorganismos sometidos a situaciones de estrés. El objetivo de este estudio era evaluar el funcionamiento de las placas Petrifilm™ and SimPlate™ en la enumeración total de aerobios y hongos (levaduras y mohos) en zumos de frutas ácidas, usando zumo de naranja no pasteurizado como modelo experimental. Las muestras se analizaron antes y después de la neutralización del pH y los resultados se compararon con los obtenidos usando métodos convencionales, como el de vertido en placa agar nutritivo estándar, y el cultivo en placas con agar glucosado de patata acidificado y con agar diclorán-glicerol. Los resultados obtenidos con Petrifilm y con SimPlate en el recuento de aerobios mesófilos y en el de levaduras y mohos se correlacionaban bien con los resultados obtenidos usando métodos convencionales. Aunque no se apreciaron diferencias estadísticamente significativas entre los recuentos de las muestras neutralizadas y las que no lo estaban (α ≤ 0.05), en las muestras neutralizadas se observaron mejores índices de correlación. Ambos sistemas (Petrifilm and SimPlate) demuestran ser buenos métodos alternativos para analizar la calidad microbiológica de los zumos de frutas ácidas. [Int Microbiol 2005; 8(1):49-53] Palabras clave: análisis microbiológico · zumos de frutas ácidas · métodos rápidos · Petrifilm™· SimPlate™ | Comparação do desempenho de sistemas prontos-para-uso utilizados na determinação da qualidade microbiológica de sucos de frutas ácidas, empregando suco de laranja não pasteurizado como modelo experimental Resumo. Atualmente, existem vários métodos alternativos para análise microbiológica rápida de alimentos. Devido à suas muitas vantagens, especialmente a suaconveniência de uso, a utilização de sistemas prontos-para-uso para enumeração de microrganismos indicadores de higiene vem aumentando. Entretanto, o desempenho desses sistemas para contagem de microrganismos injuriados não é bem conhecido. Esse estudo objetivou avaliar o desempenho de placas Petrifilm™ e SimPlate™ para contagem de aeróbios totais e fungos (bolores e leveduras) em sucos de frutas ácidas, empregando suco de laranja não pasteurizado como modelo experimental. As análises foram efetuadas antes e após a neutralização do pH das amostras, e os resultados foram comparados a aqueles obtidos através do método convencional de semeadura em placas de ágar padrão para contagem, ágar batata dextrose acidificado e ágar dicloran-glicerol. Os resultados obtidos com Petrifilm e SimPlate para contagem de aeróbios e para bolores e leveduras correlacionaram-se bem com aqueles obtidos através dos métodos convencionais. Embora não houvesse diferença estatisticamente significativa entre as contagens obtidas para as amostras neutralizadas e não neutralizadas (α ≤ 0.05), os índices de correlação para as amostras neutralizadas foram mais elevados. Ambos sistemas prontos-para-uso (Petrifilm e SimPlate) foram considerados boas alternativas para a determinação da qualidade microbiológica de sucos de frutas ácidas. [Int Microbiol 2005; 8(1):49-53] Palavras chave: análise microbiológica · sucos de frutas ácidas · métodos rápidos · Petrifilm™· SimPlate™ |
Introduction
The spoilage of acidic foods is most often due to contamination with aerobic acid-tolerant bacteria as well as yeasts and molds. Thus, enumeration of these microorganisms is an important aspect of evaluating the microbiological quality of acidic foods. However, the most frequently used analytical method for enumeration, pour-plating in non-selective and selective culture media [18,30], is both time-consuming and labor-intensive [12]. For these reasons, several alternative analytical methods that are faster, more convenient, more sensitive, and more specific than conventional assays have been recently developed. Two ready-to-use systems for bacterial counting, Petrifilm™ (3M Microbiology, St. Paul, MN, USA) and SimPlate™ plates (Biocontrol Systems, Bellevue, WA, USA), are the most popular because they do not need expensive equipment nor do they require culture media preparation and labware sterilization.
Petrifilm aerobic count (AC) plates were developed in the early 1980s for the enumeration of total aerobes in foods. The plates consist of a double-film system in which the lower film is coated with dehydrated nutrients and water-soluble gelling agents, and the upper film with gelling substances and dying agents. For inoculation, the two films are separated, the sample is transferred to the center of the lower film, the upper film is repositioned, and a plastic spreader is applied to the top. After gelling, plates are incubated and the dyed colonies are counted. Several types of 3M Petrifilm plates are available for the enumeration of aerobes, coliforms, Escherichia coli, Enterobacteriaceae, Staphylococcus aureus, and yeasts and molds, and these have been extensively tested with many types of food matrices [1,6-10,13,14,16, 17,19,20,21,26,27-29,32].
The SimPlate system is based on a multiple-enzyme technology that allows enzyme activity to be correlated with the presence of viable microorganisms in food. The system consists of dehydrated medium and a plastic plate with 84 wells. The medium contains multiple substrates, and microbial detection occurs when the color of these substrates changes due to microbial hydrolysis. For testing, the food sample and reconstituted medium are dispensed onto the center of the SimPlate plate and then distributed into the wells. After incubation, positive wells are counted, and the most probable number (MPN) of colony-forming units (CFU)/ml of sample is calculated using the provided MPN conversion table. The SimPlate system has also been positively evaluated for testing a wide variety of food matrices [11,15,22,31].
Despite the widespread use of Petrifilm and SimPlate plates for the microbial monitoring of foods, there is little information regarding the performance of these two systems, especially the more recent SimPlate method, in testing foods with low pH, such as fruit juices. Microbial stress, such as caused by low pH and refrigeration, may affect the efficiency of these plates in the enumeration of hygiene indicator microorganisms, such as total aerobes, coliforms, and fungi.
The objective of the present study was to evaluate the performances of Petrifilm and SimPlate plates and to compare them with conventional methods for the enumeration of mesophilic aerobes and molds and yeasts in acidic fruit juices, before and after neutralization of pH, using freshly squeezed, non-pasteurized orange juice as the food model. Knowledge of the degree of equivalence between conventional and modern alternative analytical methods is important for establishing microbiological standards for these foods.
Material and methods
For this study, 61 samples of freshly squeezed non-pasteurized orange juice, bottled in plastic vials and with no preservatives added, were purchased in retail stores in the city of São Paulo, SP, Brazil. Samples were transported under refrigeration to the laboratory, where they were tested immediately. After homogenization of the content by manual shaking, the caps were disinfected with 70% alcohol, punctured with sterile tweezers, and 30-ml portions of juice were transferred into three different sterile flasks. One flask was used to measure the pH of the juice and to calculate the amount of 1 N NaOH needed for neutralization. The same amount of 1 N NaOH was added to the juice in the second flask. The third flask was used for analysis of non-neutralized juice. Decimal dilutions of the samples to be tested up to 1:1000 were prepared using a sterile phosphate solution [2].
Aerobic mesophilic microorganism counts. From each dilution of neutralized and non-neutralized samples, 1-ml aliquots were plated in duplicate on Petrifilm AC plates following the method recommended by the manufacturer. After incubation at 30 ± 1ºC for 48 ± 3 h, plates containing 25-250 red colonies were selected, the colonies were counted, and the average number of CFU/ml was determined.
SimPlate TPC plates were inoculated according to the procedure described by the manufacturer, using both neutralized and non-neutralized samples. Juice dilutions were used to rehydrate the culture medium, which was poured onto the plates. After incubation at 30ºC ± 1ºC for 24 ± 3h, the number of fluorescent wells was counted using a UV light source provided by the manufacturer. The MPN/ml was calculated using the MPN table also supplied by the manufacturer.
Duplicates of each dilution (1 ml) of neutralized and non-neutralized samples were pour-plated using Standard Methods Agar (Oxoid, Basingstoke, Hampshire, England) and incubated at 30 ± 1ºC for 48 ± 3 h. Plates containing 25-250 colonies were selected and counted, and the average number of CFU/ml was calculated.
Acid-tolerant mold and yeast counts. For these determinations, only non-neutralized samples were tested. One-ml aliquots of each sample dilution were plated in duplicate on Petrifilm YM plates following the manufacturer's instructions. These plates were incubated at 25 ± 1ºC for 72 ± 3 h for acid-tolerant mold and yeast counts. Plates containing 25-100 colonies were selected and counted, and the average number of CFU/ml was calculated.
SimPlate YM plates were inoculated according to the procedure described by the manufacturer. Juice dilutions were used to rehydrate the culture medium, which was poured onto the plates. After incubation at 25 ± 1ºC for 24 ± 3h, the number of fluorescent wells was counted using a UV light source provided by the manufacturer. The MPN/ml was calculated using the MPN table also supplied by the manufacturer.
One-ml aliquots of each dilution of non-neutralized samples were plated in duplicate onto potato dextrose acidifed agar (PDA) and dichloran-glycerol agar (DG-18), both from Oxoid. Plates were incubated at 25 ± 1ºC for 72 ± 3 h and those containing 25-100 colonies were selected. The colonies were counted and the average number of CFU/ml was calculated.
Statistical analysis. Values obtained from the counts were converted to logarithmic form and the correlation coefficient, slope, and intercept for the results obtained by the three assays were calculated using linear regression methods (α ≤ 0.05) and variance analysis (t-test, α ≤ 0.05) using MS Excel 7.0 (Microsoft Inc., USA). Correlations were ranked as "excellent", "good", or "fair" according to the values of the correlation indexes, as shown in Table 1.
Results and Discussion
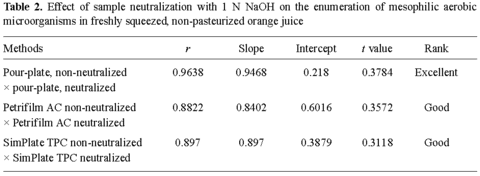
The correlation indexes (r) calculated for the enumeration of mesophilic aerobes in neutralized and non-neutralized orange juice samples using conventional pour-plates, Petrifilm AC plates, and Simplate TPC plates are presented in Table 2. As shown, the conventional pour-plating method resulted in the highest r value (0.9638), followed by SimPlate TPC plates (r = 0.8970), and by Petrifilm AC plates (r = 0.8822). The differences observed between the results of neutralized and non-neutralized samples were not significant (α < 0.05). These data indicate that neutralization of the pH of the juice had little effect on the performance of these analytical methods.
When counts of mesophilic aerobes obtained by conventional pour-plating in Standard Methods Agar were compared to those obtained using Petrifilm AC plates, the correlation indexes were higher for neutralized samples than for non-neutralized samples. While the first correlation ranked "fair", the second ranked "good" (Table 3). Despite this, the differences were not significant (α < 0.05).
The r values obtained when Petrifilm AC was compared to the conventional plating method were lower than those reported by other authors for neutral-pH foods of animal origin [1,6-10,13,14,17,19,20,21,26,27-29,32]. In addition, previous studies carried out in Brazil with bovine and caprine milk have shown that correlation indexes may be lowered by the interference of microorganisms from the autochthonous microflora, which are unable to degrade the color indicator of the Petrifilm AC plates [3].
As for SimPlate TPC plates, correlation indexes were ranked "fair" in non-neutralized samples, but "excellent" in neutralized samples (Table 3). In other studies, the correlation indexes obtained by analyzing a wide range of foods and water were higher than in the present study [5,11,15,22,31]. In addition, previous studies carried out by Silva and Gallo [23] and Beloti et al. [3] in Brazil yielded lower correlation indexes than those reported here.
When molds and yeast were counted, correlations indexes between results obtained from traditional culture methods using acidified PDA and those from Petrifilm YM plates were ranked "excellent" (Table 4). Beuchat et al. [4, 6] and Knight et al. [17] also obtained high correlationindexes for acidic products assayed on acidified PDA and Petrifilm YM plates. Note that, when DG-18 agar, recommended for the enumeration of xerophilic molds, was used instead of acidified PDA, the correlation indexes ranked only "fair". This could have been due to the lack or scarcity of xerophilic molds in orange juices. By contrast, yeasts tend to be more abundant because they can tolerate low pHs and frequently can grow in anoxic media, whereas molds are strictly aerobic.
Correlation indexes obtained for mold and yeast counts using acidified PDA and SimPlate YM plates also ranked "excellent" (Table 4). Again, when DG-18 agar was used instead of acidified PDA, the correlation indexes were ranked as "fair". In analyses of mold and yeast in foods, Spangenberg and Ingham [24] and Taniwaki et al. [27] showed that the use of SimPlate did not result in correlation indexes as high as those reported in previous studies from our laboratory [5,31]. The correlation coefficients measured by Spangenberg and Ingham [24] ranged from 0.88 to 0.90, while those of Taniwaki et al. [27] ranged from 0.61 to 0.69 in a comparison of SimPlate, conventional methods, and Petrifilm, whereas for Petrifilm the correlation coefficient was 0.9299 in a comparison with conventional pour-plates.
The results of this study indicate that both ready-to-use systems are good alternatives for the enumeration of hygiene indicator microorganisms in acidic fruit juices and that neutralization of the pH of the food sample is not required when using these systems. In addition, regardless of the method used, the counts of aerobic, mesophilic microorganisms in the juice samples were high: in 61% of the samples the counts were over 104 CFU/ml. The counts of molds and yeasts were also high: in 50% of the samples they surpassed 104 CFU/ml. The availability of inexpensive small juicers has increased the popularity in hot tropical climates of freshly squeezed, non-pasteurized, refrigerated orange juice, such as is sold on the streets and in all kinds of vending places in Brazil [25]. However, the results of this study indicate that freshly squeezed non-pasteurized orange juice may contain a very high load of microorganisms and should thus be of concern to local health authorities.
Acknowledgements. The authors are grateful to Conselho Nacional de Pesquisa (CNPq) for financial support and to Coordenadoria de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the graduate fellowship to author ARF.
References
1. Abgrall B, Cleret JJ (1990) Evaluation of Petrifilm™ SM for the enumeration of the aerobic flora of fish. J Food Prot 53:213-216 [ Links ]
2. Andrews WH, Hammack TS (2003) Food sampling and preparation of sample homogenate. In: Bacteriological analytical manual online, 8th edn. Center for Food Safety & Applied Nutrition, US Food and Drug Administration. Available online [http://www.cfsan.fda.gov/~ebam/bam-1.html], last visited Dec. 3, 2004 [ Links ]
3. Beloti V, Barros MAF, Nero LA, Pachemshy JAS, Santana EHW, Franco BDGM (2002) Quality of pasteurized milk influences the performance of ready-to-use systems for enumeration of aerobic microorganisms. Intern Dairy J 12:413-418 [ Links ]
4. Beuchat LR, Nail BV, Brackett RE, Fox TL (1991) Comparison of the Petrifilm™ yeast and mold culture film method for enumerating yeasts and mold in foods. J Food Prot 54:443-447 [ Links ]
5. Beuchat LR, Copeland F, Curiale MS, Danisavich T, Gangar V, King BW, Lawlis TL, Likin RO, Okwusoa J, Smith CF, Townsend DE (1998) Comparison of the SimPlate total count method with Petrifilm, Redigel, and conventional pour-plate methods for enumerating aerobic microorganisms in foods. J Food Protect 61:14-18 [ Links ]
6. Beuchat LR, Neil BV, Brackett RE, Fox TL. (1990) Evaluation of a culture film (Petrifilm YM) method for enumerating yeasts and molds in selected dairy and high-acid foods. J.Food Prot 53:864, 869-874 [ Links ]
7 Bishop JR, Juan JY (1988) Improved methods for quality assessment of raw milk. J Food Prot 51:955-957 [ Links ]
8. Curiale MS, Fahey P, Fox TL, McAllister JS (1989) Dry rehydratable films for enumeration of coliforms and aerobic bacteria in dairy products: collaborative study. J Assoc Off Anal Chem 72:312-318 [ Links ]
9. Ellis P, Meldrum R (2002) Comparison of the compact dry TC and 3M petrifilm ACP dry sheet media methods with the spiral plate method for the examination of randomly selected foods for obtaining aerobic colony counts. J Food Prot 65:423-425 [ Links ]
10. Erdmann, JJ, Dickson, JS, Grant, MA. (2002) A new technique for Escherichia coli testing of beef and pork carcasses. J Food Prot 65:192-195 [ Links ]
11. Feldsine PT, Leung SC, Lienau AH, Mui LA, Townsend DE (2003) Enumeration of total aerobic microorganisms in foods by SimPlate total plate count-color indicator methods and conventional culture methods: collaborative study. J AOAC Int 86:257-274 [ Links ]
12. Feng P (2001) Rapid Methods for Detecting Foodborne Pathogens. In: Bacteriological analytical manual online, 8th edn. Appendix 1, Center for Food Safety & Applied Nutrition, US Food and Drug Administration. Available online [http://www.cfsan.fda.gov/~ebam/bam-a1.html], last visited on Dec. 15, [ Links ] 2004
13. Ginn RE, Packard VS, Fox TL (1986) Enumeration of total bacteria and coliforms in milk by dry rehydratable film methods: collaborative study. J Assoc Off Anal Chem 69:527-531 [ Links ]
14. Ginn RE, Packard VS, Fox TL (1984) Evaluation of the 3M dry medium culture plate (Petrifilm™ SM) method for determining numbers of bacteria in raw milk. J Food Prot 47:753-755 [ Links ]
15. Jackson RW, Osborne K, Barnes G, Jolliff C, Zamani D, Roll B, Stillings A, Herzog D, Cannon S, Loveland S (2000) Multiregional evaluation of the SimPlate heterotrophic plate count method compared to the standard plate count agar pour plate method in water. Appl Environ Microbiol 66:453-454 [ Links ]
16. Jordano R, Lopez C, Rodriguez V, Cordoba G, Medina LM, Barrios J (1995) Comparison of Petrifilm method to conventional methods for enumerating aerobic bacteria, coliforms, Escherichia coli, yeast and molds in foods. Acta Microbiol Immunol Hung 42:255-259 [ Links ]
17. Knight MT, Newman MC, Benzinger MJ, Neufang KL, Agin JR, McAllister JS, Ramos M (1997) Comparison of the Petrifilm dry rehydratable film and conventional culture methods for enumeration of yeasts and molds in foods: collaborative study. J AOAC Int 80:806-811 [ Links ]
18. Maturin LJ, Peeler JT (2001) Aerobic plate count. In: Bacteriological analytical manual online, 8th edn. Center for Food Safety & Applied Nutrition, US Food and Drug Administration. Available online [http://www.cfsan.fda.gov/~ebam/bam-3.html], visited on Dec. 15, 2004 [ Links ]
19. McAllister JS, Ramos MS, Fox TL (1987) Evaluation of the 3M dry medium culture plate (Petrifilm™ SM) method for enumerating bacteria in processed fluid milk samples. Dairy Food Sanit 7:632-635 [ Links ]
20. Park YH, Kalantari A, Frank JF (2004) Changes in the microflora of commercial goat milk cheese during refrigerated and frozen storage. Small Ruminant Res 53:61-66 [ Links ]
21. Park YH, Seo KS, Ahn JS, Yoo HS, Kim SP (2001) Evaluation of the Petrifilm plate method for the enumeration of aerobic microorganisms and coliforms in retailed meat samples J Food Prot 64:1841-1843 [ Links ]
22. Russell SM (2000) Comparison of the traditional three-tube most probable number method with the Petrifilm, SimPlate, BioSys optical, and Bactometer conductance methods for enumerating Escherichia coli from chicken carcasses and ground beef. J Food Prot 63:1179-1183 [ Links ]
23. Silva MC, Gallo CR (2003) Avaliação da qualidade microbiológica de alimentos com a utilização de metodologias convencionais e do sistema Simplate. Higiene Alimentar 17:75-85 (in Portuguese) [ Links ]
24. Spangenberg DS, Ingham SC (2000) Comparison of methods for the enumeration of yeasts and molds in shredded low-moisture, part skim mozzarella cheese. J Food Prot 63:529-533 [ Links ]
25. Sugai AY, Shigeoka DS, Badolato GG et al. (2002) Physico-chemical and microbiological analyses of minimally processed orange juice stored in aluminium cans. Cienc Tecnol Alim 22:529-533 [ Links ]
26. Suwansonthichai S, Rengpipat S (2003) Enumeration of coliforms and Escherichia coli in frozen black tiger shrimp Penaeus monodon by conventional and rapid methods. Int J Food Microbiol 81:113-121 [ Links ]
27. Taniwaki MH, Silva N, Banhe AA, Iamanaka BT (2001) Comparison of culture media, Simplate, and Petrifilm for the enumeration of yeasts and molds in food. J. Food Prot 64:1592-1596 [ Links ]
28. Tessi MA, Aringoli EE, Pirovani ME et al (2002) Microbiological quality and safety of ready-to-eat cooked foods from a centralized school kitchen in Argentina. J Food Prot 65:636-642 [ Links ]
29. Thunberg RL, Tran TT, Bennett RW, Matthews RN, Belay N. (2002) Microbial evaluation of selected fresh produce obtained at retail markets. J Food Prot 65:677-682 [ Links ]
30. Tourna V, Stack ME, Mislivec PB, Koch HA, Bandler R (2001) Yeasts, molds and mycotoxins. In: Bacteriological Analytical Manual Online, 8th ed. Center for Food Safety & Applied Nutrition, US Food and Drug Administration. Available online [http://www.cfsan.fda.gov/~ebam/bam-18.html], last visited on Dec. 15, 2004 [ Links ]
31. Townsend DE, Naqui A (1998) Comparison of SimPlate™ total plate count test with plate count agar method for detection and quantitation of bacteria in food. J AOAC Int 3:563-569 [ Links ]
32. Vlaemynck GM (1994) Comparison of Petrifilm™ and plate count methods for enumeration molds and yeasts in cheese and yogurt. J Food Prot 57:913-914 [ Links ]












 Curriculum ScienTI
Curriculum ScienTI