Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
International Microbiology
versão impressa ISSN 1139-6709
INT. MICROBIOL. vol.8 no.3 Set. 2005
| RESEARCH REVIEW | |||
|
| |||
| Contributions of in situ microscopy to the current understanding of stone biodeterioration
Summary. In situ microscopy consists of simultaneously applying several microscopy techniques without separating the biological component from its habitat. Over the past few years, this strategy has allowed characterization of the biofilms involved in biodeterioration processes affecting stone monuments and has revealed the biogeophysical and biogeochemical impact of the microbiota present. In addition, through in situ microscopy diagnosis, appropriate treatments can be designed to resolve the problems related to microbial colonization of stone monuments. [Int Microbiol 2005; 8(3):181-188] Key words: biodeterioration · in situ microscopy · biofilms · scanning electron microscopy in back-scattered mode · lithobionts | ||
| |||
Contribuciones de la microscopia in situ a la comprensión actual del biodeterioro de las rocas Resumen. La microscopia in situ consiste en aplicar simultáneamente varias técnicas de microscopia sin separación de los componentes biológicos de su hábitat en la roca. Durante los últimos años, esta estrategia ha permitido caracterizar las biopelículas implicados en los procesos del biodeterioro que afectan los monumentos de piedra y ha revelado el impacto biogeofísico y biogeoquímico de la microbiota presente. Además, el diagnóstico mediante microscopia in situ permite diseñar tratamientos apropiados para resolver los problemas relacionados con la colonización microbiana de los monumentos de piedra. [Int Microbiol 2005; 8(3):181-188] Palabras clave: biodeterioro · microscopia in situ · biopelículas · microscopia electrónica de barrido en modo back-scattered · litobiontes | Contribuições da microscopia in situ à comprensão atual do biodeterioro das rochas Resumo. A microscopia in situ consiste em aplicar simultaneamente várias técnicas de microscopia sem separação dos componentes biológicos de seu hábitat na rocha. Durante os últimos anos, esta estratégia permitiu caracterizar os biofilmes implicados nos processos do biodeterioro que afetam os monumentos de pedra e revelou o impacto biogeofísico e biogeoquímico da microbiota presente. Além disso, o diagnóstico mediante microscopia in situ permite desenhar tratamentos apropriados para resolver os problemas relacionados com a colonização microbiana dos monumentos de pedra. [Int Microbiol 2005; 8(3):181-188] Palavras chave: biodeterioro · microscopia in situ · biofilmes · microscopia eletrônica de varredura em modo back-scattered · lithobiontes |
Introduction
For thousand of years, when grandeur and beauty were the goals of architecture, stone was the most widely used durable material. The deterioration of building stone thus causes causing irreparable loss to our cultural heritage [29]. Besides chemical and physical factors, microorganisms play a major role in rock decay. In fact, microorganisms can modify the rates and mechanisms of chemical and physical weathering of rocks [11]. Through recognition and understanding of deterioration processes induced by microorganisms, we will be able to solve some of these associated problems and thereby prevent further damage to monuments. To this end, there is a need for detailed information not only on the type, intensity, and extent of weathering damage, but also on the distribution and specific effects of the different microorganisms and organisms colonizing monuments [47]. However, this information is difficult to obtain, since the microbiota of rocks are embedded in a hard and opaque substrate, forming a complex community of organisms capable of weathering stone while establishing many interrelationships. Microscopy is the most suitable tool for analyzing such biodeterioration processes, especially when it is applied in situ, that is, when the biological components are not separated from the lithic material that arrives at the laboratory.
Microscopy techniques to study microorganisms-rock interfaces
Several microscopy techniques have been used to study microorganism-rock interfaces [5]. The first investigations of the lichen-rock relationship using non-destructive techniques were carried out with light microscopy [3] and light microscopy combined with scanning electron microscopy [4,26,37,46]. However, it was not until the early 1990s, with the development of scanning electron microscopy in back-scattered mode (SEM-BSE) technique [48], that interactions between microorganisms and rock lithic substrates were precisely examined, since with this technique all organic and mineral phases could be jointly analyzed (in situ) with good resolution. In SEM-BSE, fixed rock fragments containing biological material embedded in resin and then polished are analyzed. Note that the microorganisms involved in the deterioration of rock monuments are not only those we can externally recognize on the monument rock (epilithic) but also those present inside the rock (endolithic). As with light microscopy, SEM-BSE technique allows the examination of an area of several square centimeters, but with a higher resolution, more similar to that of transmission electron microscopy (TEM). The increased resolution allows epilithic and endolithic microorganisms as well as nearby minerals to be simultaneously visualized [48].
Recently, SEM-BSE was complemented with other in situ microscopy techniques, such as low-temperature SEM (LTSEM) and confocal scanning laser microscopy (CSLM), which have allowed compilation of a complete picture showing all aspects of the colonizing microbial communities involved in biodeterioration processes [6,9,12]. CSLM provides mainly spatial information on microbial colonization while LTSEM provides data related to physical effects. In the last few years, environmental SEM (ESEM) has also been used to analyze biodeterioration processes [16,38]. Both ESEM and LTSEM enable hydrated, non-conducting samples to be viewed with minimum handling. However, LTSEM provides more information on the presence of water and extracellular polymeric substances (EPS), since fresh fractures in frozen samples can be examined. Furthermore, the use of SEM-BSE in conjunction with X-ray energy dispersive spectroscopy (EDS) has been indispensable for characterizing the biochemical actions of microorganisms [6,17,45]. A combined SEM-EDS set-up was first used by Halbauer and Jahns in the 1970s [26]. However, because of the poor development stage of those techniques at that time, EDS was only used to discern between organic and inorganic components. It was not until the advent of SEM-BSE technique [48] that the most interesting information on biodeterioration processes became accessible [7,9,10,18,39]. The combination TEM-EDS has been also of great use for observing mineral deposits associated with microbial cells [4], and high-resolution TEM (HRTEM) has emerged as an efficient tool for demonstrating mineralogical transformations on the nanoscale [49].
Microbiota of monumental rocks
Several types of microorganisms have been found to colonize monument stone. Lichens (the symbiotic association of fungi with photosynthesizing microorganisms algae and/or cyanobacteria) and some colored films corresponding to free-living cyanobacteria and green algae can be identified by directly observing the rock surface. However, this is not always possible for free-living fungi and heterotrophic bacteria, which frequently require the use of microscopy for their detection. Through in situ microscopy, the zones where biodeterioration processes take place have been visualized as biofilms composed of different microorganisms, the organic compounds these microorganisms generate, and mineral and inorganic compounds [9,18].
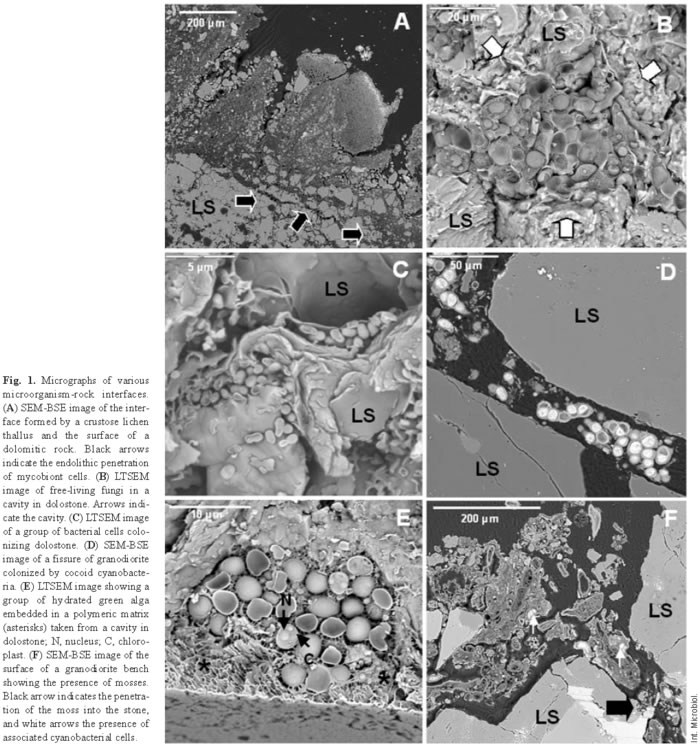
Lichens are regarded as primary colonizers and very aggressive agents in biodeterioration [31]. Figure 1A shows the surface of a dolomitic rock colonized by lichen thalli. SEM-BSE reveals the interface between the lichen and the underlying substrate, and also how the symbiont cells penetrate the rock (black arrows), causing extensive disaggregation of the dolomitic rock. Endolithic microbial colonization seems to be induced by enhanced moisture availability in cracks and fissures. The presence of endolithic lichen symbionts in monument stones and their role in biodeterioration have been revealed only with the emergence of these new in situ microscopy techniques. In fact, conventional techniques generally applied to biodeterioration studies, such as microbiological cultures and light microscopy, provide information only about the microorganisms, but not about their relationship with the stone [42].
In addition to lichenized fungi, other fungi have been also detected in monument stone, such as the fungus embedded in a mineral matrix, shown in Fig. 1B (arrows). Fungal types associated with monument stone vary considerably [27]. In this regard, it is now widely accepted that rock surfaces are common habitats for chemoorganotrophic and chemolithotrophic bacteria, actinomycetes, and especially fungi [30,44]. Heterotrophic bacteria are frequently observed in close association with the rest of the microorganisms in the altered zone [9,18], but they may also form independent colonies (Fig. 1C).
In addition to lichen photobionts, other phototrophic microorganisms can colonize monument rock. Free-living cyanobacteria and algae have been observed on rock surfaces [2,33,35,39] and inside fissures and cavities in carbonate [7,9,34,40] and granite rocks [18]. Figure 1D is an SEM-BSE image showing a fissure in a granodiorite rock colonized by coccoid cyanobacteria. Figure 1E shows a hydrated group of algal cells occupying a cavity in the lower surface of a detached dolostone, as fragment visualized by LTSEM. In these cells, the nucleus and chloroplast can be distinguished, showing the ultrastructural resolution ability of this technique.
Mosses commonly appear also in the proximity of microorganisms colonizing lithic substrates. Figure 1F shows mosses at a granodiorite bench. Cyanobacterial cells were observed associated with the moss cells (white arrows). The trapping of atmospheric dust by mosses can favor the presence of associated microbiota and also lead to the formation of enough substrate so that other plants can grow there [31].
Fungi, either free-living or as lichen symbionts, were the most abundant microorganisms found in the monuments that we analyzed. Fungi have ecological advantages over bacteria and algae as lithobionts due to their higher tolerance of low water potential, their effective modes of propagation and reproductive strategies, and their ability to thrive (and propagate) even at poor nutrient concentrations [27]. Structural and functional interactions between the different components of the system must be established because the biofilm remodels itself in a way that is dependent on internal interactions between different microorganisms [20]. In this way, the presence of different microorganisms might be favored by the ability of epilithic lichens to form microenvironments in rock substrates [17]. In turn, phototrophic microorganisms support the growth of other microorganisms, such as fungi or bacteria, with higher chemical destructive potential [34].
Effects of microbiota on monument rocks
A common problem in research on biodeterioration is the difficulty in distinguishing between physical and chemical weathering caused by abiotic agents and that caused by biotic agents [14]. Endoliths cause alterations to the stone surface that resemble the effects of an abiotic origin, and visual examination often reveals no evidence of biological colonization [42]. The bioweathered zone is a complex organic-mineral interface, which must be correlatively examined to identify the true processes acting on it and the microorganisms involved in the different chemical and physical effects observed.
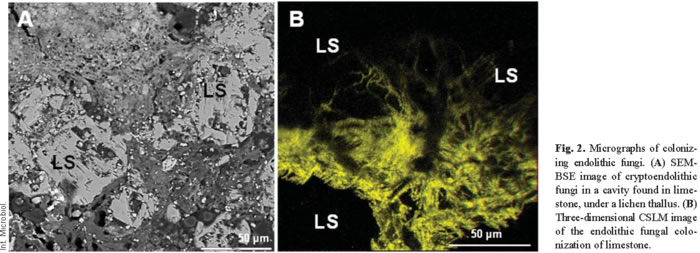
In situ microscopy of vertically fractured samples has allowed investigators to observe the development of microorganisms on the surface of the samples and has revealed numerous details of their penetration into the substrate. The penetration of growing microorganisms into the rock, and the diffusion of their excreted products into intergranular fissures probably enhance weathering reactions and decrease cohesion between grains. The action of biofilms on rocks has two different components, physical and chemical. Physical actions are erosion and the breaking of surface layers due to microbial adhesion and penetration between particles of the substrate. Chemical actions include dissolution and chelating processes involved in the formation of metal complexes incurred by metabolic products and other substances produced by microorganisms [32]. SEM-BSE micrographs reveal the potential mechanical impact of biofilms (Figs. 1D,F and 2A). Physical alterations are the result of two main effects. First, effects can be attributable to an increased mass of microorganisms as they grow. This growing effect can be observed in Fig. 1F. Penetration of the moss (black arrow) probably produced intense pressure on the lithic substrate. This effect is also clear from the fungal penetration shown in Fig. 2A. CSLM showed this penetration in three dimensions, giving a better idea of the pressures generated in the network of the fissures in the rock (Fig. 2B). The yellow hyphae appear against a black background, which corresponds to the granite substrate. Second, physical pressures can also arise from the water-binding capacity of microorganisms, whereby their volume changes in wet and dry periods and winter frosts due to repeated extension and shrinkage of the colony. These effects are well-established by LTSEM (Fig. 1B,E). Alterations related to the presence of water can be discerned by LTSEM, which shows microorganisms in several states of hydration and the location of water in a hydrated sample [9]. The most intense physical actions are often those produced by fungi. Lichenized fungal cells penetrate the spaces between rock grains and along mineral exfoliation planes. This penetration, along with growth of the fungus within the substrate (Figs. 2A,B), leads to rock fragmentation. This is a common phenomenon observed in several types of rock, such as limestone [8,9,18], marble [32], granite [5,17,43], or sandstone [28]. Black fungi have also been noted to cause physical damage to stone due to their efficient rock-penetrating capacity [19,25]. Physical damage caused by poikilotroph microcolonial micromycete fungal biofilms in rocks was recently demonstrated by microscopy and characterization of the physical properties of these colonies [20].
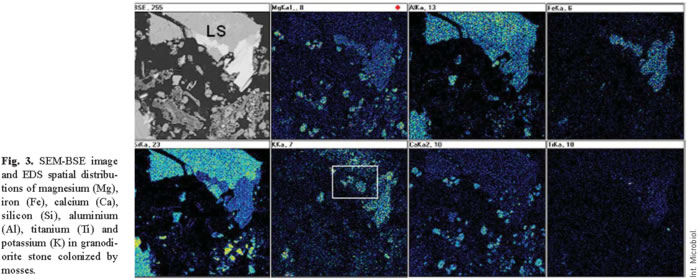
The combined effects of chemical and physical action also cannot be precluded. Indeed, chemical changes may be concurrent with the physical disintegration of rock [1]. The release of organic acids exerts a major chemical effect on substrates. SEM or TEM combined with EDS microanalysis is indispensable for an evaluation of such effects. Figure 3 shows the distribution maps for the different elements detected in a substrate zone colonized by mosses and cyanobacteria. Depletion of potassium in some of the mica layers is readily seen (see box). Similar biomobilization phenomena have been attributed to several lichens, both in monument and natural stone [17,36,49], and to cyanobacteria colonies [18]. In bioweathered materials, secondary products (biominerals) can arise from dissolving and chelating microbial actions [49]. These biomineralization processes are frequently observed also in the proximity of the lithic microbiota of monuments.
Extracellular calcium carbonate deposits have been attributed to the presence of cyanobacteria in the biodeterioration of the Tower of Belem in Lisbon, Portugal [7]. Calcification of cell walls and biomineralization processes in carbonate nodules have been linked to the microbiota present in the Roman Cathedral of Jaca, Spain [8]. Calcium oxalate has been also observed in lichen-colonized monument rocks [10]. Figure 1F shows, among phylidium moss, calcium-rich minerals resulting from the biomineralization process. High calcium levels in plants have been related to the damage they inflict on calcareous rocks [31]. Biomineralization processes, the deposition of clay and quartz particles from the environment and, in polluted areas, also the deposition of toxic pollutants have been implicated in the formation of aesthetic patinas on monuments [22,30,35].
Microbiota that cause biodeterioration processes
Note that biodeterioration processes in a given site are rarely caused by only one distinct group of microorganisms. Any biodeterioration process is probably the result of complex microbial interactions [47]. Lichens have been described as major biodeterioration agents [1,15,31]. Some authors consider black meristematic fungi as very harmful microorganisms that contribute to the biodeterioration of monuments [19,25]. The actions of other epilithic and endolithic microorganisms may be less severe but nonetheless very harmful. The presence and close association of phototropic microorganisms and heterotrophic bacteria with the altered lithic substrate also suggest their participation in the decay process [7,9,12,33,34]. Physical damage produced by other microorganisms is less than that caused by more aggressive fungi. Nonetheless, the chemical contributions of free-living phototropic microorganisms and heterotrophic bacteria are significant, since they produce and secrete a variety of potentially damaging metabolic products [23]. In addition, the extracellular polymeric matrix associated with the biological components of the biofilm produce specific effects. Extracellular polymeric substances (EPS) can be observed in all biofilms and are integral components of their structural organization. In Fig. 1E, a group of algae embedded in a polymeric matrix (asterisks) inside a rock cavity can be clearly visualized. Although the amount of EPS depends on the type of biofilm and its location, the matrix is always an essential component of the biofilm, both at epilithic and at endolithic sites. In complex communities, the extracellular matrix comprises the EPS produced by the different resident microorganisms. Biologically initiated chemical effects on rocks are frequently connected to the presence of EPS that act as sorbents of metal ions. However, EPS actions have to be interpreted also in relation to the physical damage, since this component undergoes substantial volume changes during cycles of drying and rewetting and of freezing and thawing; this, in turn, causes loosening of the stone grains. The hygroscopic properties of EPS and the consequent higher water content of the microenvironment around microbial communities may also enhance nutrient availability and interactions among microorganisms [50].
Adaptive features of endolithic microorganisms
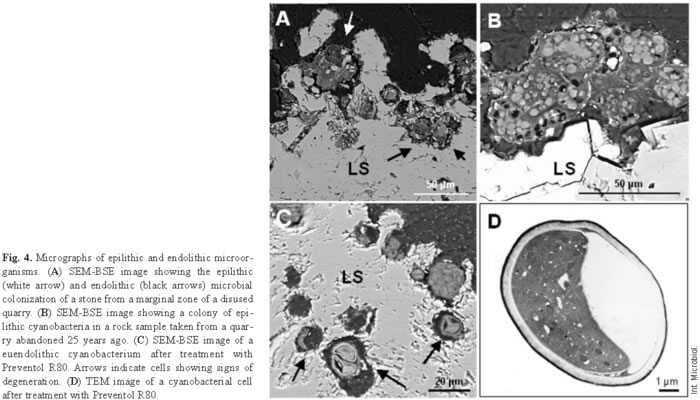
Microorganisms that live within hard rocky substrates acquire specialized adaptive features, which in turn define different endolithic ecological niches. These microorganisms colonize existing cracks and fissures (chasmoendolithic), internal pores (cryptoendolithic), or penetrate actively into the rock (euendolithic) [24]. Each lithobiontic ecological niche needs to be precisely characterized, both biologically and mineralogically, to fully understand the dynamic relationships in play. In situ microscopy studies of microbial interactions with the lithic substrate have allowed researchers to establish the ecological niches of the different microorganisms present in the biofilm. This characterization has provided insight into the roles of microorganisms in biodeterioration processes. The cyanobacteria shown in Fig. 1D are chasmoendolithic microorganisms, since they seem to be occupying pre-existing fissures in the rock. The green algae observed in Fig. 1E can be designated as cryptoendolithic because they occupy a cavity in the rock. Euendolithic cyanobacteria are seen in Fig. 4A. The effects of chemical attack on the stone are clearly visible in places where the cavity harbors the microorganisms and takes on the typical pear shape of their colony [7]. In some cases, it is difficult to attribute an euendolithic nature to the microorganisms. Some of the components of certain biofilms are cryptoendolithic (Fig. 2A), meaning that the microorganisms colonize cavities in the rock, but the inferred substrate-altering capacity of some suggests a euendolithic niche.
Microscopy analysis has also been carried out in several studies on quarry stones [10,22]. The advantage of using this type of sample is that there is no limit to the amount of material used for the different tests [22]. Studies in abandoned quarries have been useful for evaluating the time frames for the development and action of biofilms [10]. Figure 4B shows the first stages of colonization of a site in a quarry that had been in disuse since the late 1970s. These rocks showed only epilithic colonization, in this case, by cyanobacteria. Colonization was very different from that observed in rocks from the quarry limits, where epilithic and endolithic colonization was observed (Fig. 4A). Together, the findings suggest a gradual sequential colonization process in these carbonate rocks.
Evaluating treatments
Integration of all the information generated by in situ microscopy allows the design of appropriate treatment strategies [9,18]. Treatments aimed at destroying favorable conditions for growth of the most damaging microbiota or at directly inhibiting or eliminating them are some of the available options. The effectiveness of a given treatment can also be assessed through microscopy. Each treatment should be evaluated on a case-by-case basis, considering the particular features of lithic biofilms. For instance, the tolerance of biofilm microorganisms to biocides is much higher than that of suspended cells [21]. The EPS matrix and stone can diminish the penetration and diffusion of an antimicrobial agent into the biofilm, thus reducing the effectiveness of applied treatments. For this reason, treatments should first be applied on a small scale but directly on the lithic biofilms present in the monument before evaluating their effects. A few studies have been designed to evaluate different treatments. Ascaso et al. [9] compared the use of three commercial biocides to treat a wall of the Hieronymites Monastery cloister (Lisbon, Portugal). Four months after application, several untreated and biocide-treated stone fragments were analyzed and compared. The images obtained in this study showed euendolithic cyanobacteria with collapsed protoplasms and others that had completely lost their cell structure after treatment with Preventol R80 (Fig. 4C). The TEM image of Fig. 4D confirmed plasmolysis and the complete loss of cell organization in cells treated with Preventol R80. The lack of resistant forms in the rock after treatment is also important for estimating the effectiveness of the treatment. These measures must be combined with strategies designed to impair environmental conditions that will stimulate microbial colonization. Otherwise, new forms of biological colonization will arise sooner or later.
Concluding remarks
In situ microscopy diagnosis identifies the microorganisms involved in biodeterioration processes, their chemical and mechanical effects, the extent of biodeterioration, and the effectiveness of treatment. Microscopy techniques described here are suitable for stone samples taken from monuments from which only small quantities of material may be removed. Each of these techniques provides different information on a single sample, and it is through integration of all the data obtained that insight is gained into the structure, composition, and physical properties of the sample [13]. This combined approach reveals the living community as a complex interacting system, present in epilithic and endolithic sites, that is detrimental to monument stone. Effects on mineral species inside fissures and possible chemical losses and/or gains are, in some way, determined by the nature of the colonizing microbial populations [17]. Thus, it is vital that we understand the mineral transformations that occur and obtain relevant cytological information on such microorganisms. More recently, molecular biology techniques have been successfully applied to bioweathering studies [41]. In the years to come, the combination of molecular techniques and in situ microscopy will no doubt further improve our understanding of the microorganisms involved in biodeterioration processes.
The actions of the epilithic components of the biofilm on monuments are usually obvious, but the role of endolithic forms in stone biodeterioration may be underestimated in the absence of proper in situ microscopy diagnosis. Endolithic microorganisms are probably more widespread than previously thought. Improved knowledge of these microorganisms is essential for an accurate evaluation of the damage caused or for the correct planning of restoration measures [42]. In addition, the chemical composition and texture of the material determine the resistance of a building to colonization by forms of life and to atmospheric agents [31]. Hence, only when a diagnosis based on all these aspects is tailor-made for each case can an appropriate treatment strategy be designed. Incorrect treatment may result in the elimination of only certain microorganisms, which, in turn, could promote the growth of other, more aggressive organisms in the absence of competitors.
Acknowledgements. The authors would like to thank to the staff of the microscopy facilities of the Centro de Ciencias Medioambientales for technical assistance, Jacek Wierzchos (Universidad de Lleida) and M. Angeles García del Cura (Instituto de Geología Económica, CSIC-UA), for useful comments, and Ana Burton for reviewing the English. Financial support was provided by Projects PGC BOSS2003 02418 and JCL SEK 504.
References
1. Adamo P, Violante P (2000) Weathering of rocks and neogenesis of minerals associated with lichen activity. Appl Clay Sci 16:229-256 [ Links ]
2. Albertano P, Urzi C (1999) Structural interactions among epilithic cyanobacteria and heterotrophic microorganisms in Roman hypogea. Microb Ecol 38:244-252 [ Links ]
3. Ascaso C, Sancho LG, Rodriguez-Pascual C (1990) The weathering action of saxicolous lichens in maritime Antarctica. Polar Biol 11:33-39 [ Links ]
4. Ascaso C, Ollacarizqueta MA (1991) Structural relationship between lichen and carved stonework of Silos Monastery, Burgos, Spain. Int Biodet 27:337-349 [ Links ]
5. Ascaso C, Wierzchos J (1995) Study of the biodeterioration zone between the lichen thallus and the substrate. Crypto Bot 5:270-281 [ Links ]
6. Ascaso C, Wierzchos J, de los Ríos A (1998) In situ cellular and enzymatic investigations of saxicolous lichens using correlative microscopical and microanalytical techniques. Symbiosis 24:221-234 [ Links ]
7. Ascaso C, Wierzchos J, Delgado Rodrigues J, Aires-Barros L, Henriques FMA, Charola AE (1998) Endolithic microorganisms in the biodeterioration of the tower of Belem. Int Z Bauinstandsetzen 4:627-640 [ Links ]
8. Ascaso C, Wierzchos J, Castelló R (1998) Study of the biogenic weathering of calcareous litharenite stones caused by lichen and endolithic microorganisms. Int Biodet Biodeg 42:29-38 [ Links ]
9. Ascaso C, Wierzchos J, Souza-Egipsy V, de los Ríos A, Delgado Rodrigues J (2002) In situ evaluation of the biodeteriorating action of microorganisms and the effects of biocides on carbonate rock of the Jerónimos Monastery (Lisbon). Int Biodet Biodeg 49:1-12 [ Links ]
10. Ascaso C, García del Cura MA, de los Rios A (2004) Microbial biofilms on carbonate rocks from a quarry and monuments in Novelda (Alicante, Spain). In: St. Clair LL, Seaward MRD (eds) Biodeterioration of stone surfaces. Lichens and biofilms as weathering agents of rocks and cultural heritage. Kluwer Academic Press, Dordrecht, pp 79-98 [ Links ]
11. Banfield JF, Barker WW, Welch SA, Taunton A (1999) Biological impact on mineral dissolution: application of the lichen model to understand mineral weathering in the rizosphere. Proc Natl Acad Sci USA 96:3404-3411 [ Links ]
12. Barker WW, Welch SA, Chu S, Banfield JF (1998) Experimental observations of the effects of bacteria on aluminiosilicate weathering. American Mineralog 83:1551-1563 [ Links ]
13. Brown DA, Beveridge TJ, Keevil CW, Sheriff BL (1998) Evaluation of microscopic techniques to observe iron precipitation in natural microbial biofilm. FEMS Microb Ecol 26:297-310 [ Links ]
14. Carballal R, Paz-Bermúdez G, Sanchez-Biezma MJ, Prieto B (2001) Lichen colonization of coastal churches in Galicia: biodeterioration implications. Int Biodet Biodeg 47:157-163 [ Links ]
15. Chen J, Blume HP, Beyer L (2000) Weathering of rocks induced by lichen colonization-a review. Catena 39:121-146 [ Links ]
16. Chiari G, Cossio R (2004) Lichens on Wyoming sandstone: Do they cause damage? In: St Clair LL, Seaward MRD (eds) Biodeterioration of stone surfaces. Lichens and biofilms as weathering agents of rocks and cultural heritage. Kluwer Academic Press, Dordrecht, pp 99-113 [ Links ]
17. De los Ríos A, Wierzchos J, Ascaso C (2002) Microhabitats and chemical microenvironments under saxicolous lichens growing on granite. Microb Ecol 43:181-188 [ Links ]
18. De los Ríos A, Galván V, Ascaso C (2004) In situ microscopy diagnosis of biodeterioration processes occurring in the convent of Santa Cruz la Real (Segovia, Spain). Int Biodet Biodeg 54:113-120 [ Links ]
19. Diakumaku E, Gorbushina AA, Krumbein WE, Panina L, Soukharjevski S (1995) Black fungi in marble and limestones-an aesthetical, chemical and physical problem for the conservation of monuments. Sci Total Environ 167:295-304 [ Links ]
20. Dornieden T, Gorbushina AA, Krumbein WE (2000) Biodecay of cultural heritage as a space/time-related ecological situation-an evaluation of a series of studies. Int Biodet Biodeg 46:261-270 [ Links ]
21. Flemming HC (2002) Biofouling in water systems-cases, causes and countermeasures. Appl Microb Biotechnol 59:629-640 [ Links ]
22. García-Valles M, Urzí C, De Leo F, Salamone P, Vendrell-Saz M (2000) Biological weathering and mineral deposits of the Belevi marble quarry (Ephesus, Turkey). Int Biodet Biodeg 46:221-227 [ Links ]
23. Garg KL, Dhawan S, Agrawal OP (1988) Deterioration of stone and building materials by algae and lichens: a review. National Research Laboratory for Conservation of Cultural Property, Lucknow, India, pp 1-41 [ Links ]
24. Golubic S, Friedmann I, Schneider J (1981) The lithobiontic ecological niche, with special reference to microorganisms. J Sediment Res 51:475-478 [ Links ]
25. Gorbushina AA, Krumbein WE, Hamann CH, Panina L, Soukharjevski S, Wollezien U (1993) On the role of black fungi in colour change and biodeterioration of an antique marbles. Geomicrob J 11:205-221 [ Links ]
26. Halbauer DK, Jahns HM (1977) Attack of lichens on quartzitic rock surfaces. Lichenologist 9:119-122 [ Links ]
27. Hirsch P, Eckhardt FEW, Palmer RJJ (1995) Fungi active in weathering of rock and stone monuments. Can J Bot 73:1384-1390 [ Links ]
28. Knight KB, St-Clair LL, Gardner JS (2004) Lichen biodeterioration at Inscription Rock, El Morro National Monument, Ramah, New Mexico, USA. In: St. Clair L, Seaward MRD (eds) Biodeterioration of stone surfaces. Lichens and biofilms as weathering agents of rocks and cultural heritage. Kluwer Academic Press, Dordrecht, pp 129-163 [ Links ]
29. Koestler RJ (2000) When bad things happen to good art. Int Biodet Biodeg 46:259-269 [ Links ]
30. Krumbein WE (2002) Patina and cultural heritage-a geomicrobiologist's perspective. In: Kozlowski R (ed) Proceedings of the 5th European Commission Conference "Cultural Heritage Research: a Pan European Challenge", Crakow, pp 39-47 [ Links ]
31. Lisci M, Monte M, Pacini E (2003) Lichens and higher plants on stone: a review. Int Biodet Biodeg 51:1-17 [ Links ]
32. Modenesi P, Lajolo L (1988) Microscopical investigation on a marble encrusting lichen. Studia Geobot 8:47-64 [ Links ]
33. Ortega-Calvo JJ, Hernández-Marine M, Saiz-Jimenez C (1991) Biodeterioration of building materials by cyanobacteria and algae. Int Biodet Biodeg 28:165-185 [ Links ]
34. Ortega-Calvo JJ, Hernández-Mariné M, Saiz-Jiménez C (1993) Niches for phototrophic microorganisms in stone monuments. In: Guerrero R, Pedrós-Alió C, (eds) Trends in microbial ecology. Spanish Society of Microbiology, Barcelona, pp 673-675 [ Links ]
35. Ortega-Calvo JJ, Ariño X, Hernández-Marine M, Saiz-Jimenez C (1995) Factors affecting the weathering and colonization of monuments by phototrophic microorganisms. Sci Tot Environ 167:329-3411 [ Links ]
36. Prieto B, Silva B, Rivas T, Wierzchos J, Ascaso C (1997) Mineralogical transformation and neoformation in granite caused by the lichens Tephronella atra and Ochorelechia parella. Int Biodet Biodeg 40:191-199 [ Links ]
37. Purvis OW, Elix JA, Gaul KL (1987) The occurrence of copper-norstistic acid in lichens from cupriferous substrata. Lichenologist 19:193-203 [ Links ]
38. Ray R, Little B, Wagner P, Hart K (1997) Environmental scanning electron microscopy investigations of biodeterioration. Scanning 19:98-103 [ Links ]
39. Roldan M, Clavero E, Hernández-Marine M (2003) Aerophytic biofilms in dim habitats. In: Saiz-Jiménez (ed) Molecular biology and cultural heritage. A.A. Balkema Publishers, Lisse, pp. 163-169 [ Links ]
40. Saiz-Jimenez C, García -Rowe J, García del Cura MA, Ortega-Calvo JJ, Roekens E, Van Kriegen R (1990) Endolithic cyanobacteria in Maastricht limestone. Sci Total Environ 94:209-220 [ Links ]
41. Saiz-Jiménez C (2003) Molecular Biology and cultural Heritage. Balkema, Lisse. [ Links ]
42. Salvadori O (2000) Characterisation of endolithic communities of stone monuments and natural outcrops. In: Ciferri O, Tiano P, Mastromei G (eds) Of microbes and art: the role of microbial communities in the degradation and protection of cultural heritage. Plenum, London, pp 89-101 [ Links ]
43. Silva B, Prieto B (2004) Deteriorative effects of lichens on granite monuments. In: St.Clair LL, Seaward MRD (eds) Biodeterioration of stone surfaces. Lichens and biofilms as weathering agents of rocks and cultural heritage. Kluwer Academic Press, Dordrecht, pp 69-77 [ Links ]
44. Sterflinger K, Prillinger H (2001) Molecular taxonomy and biodiversity of rock fungal communities in an urban environment (Vienna, Austria). Ant Leeuw 80:275-286 [ Links ]
45. Walker, J.J., Spear, J.R. and Pace, N.R. (2005) Geobiology of a microbial endolithic community in the Yellowstone geothermal environment. Nature 434:1011-1014. [ Links ]
46. Walton DWH (1985) A preliminary study of the action of crustose lichens on rock surfaces in Antarctica. In: Siegfred WR, Cindy PR, Laws RM (eds) Antarctic nutrient cycles and food webs, Springer Verlag, Berlin, pp 180-185 [ Links ]
47. Warscheid T, Braams J (2000) Biodeterioration of stone: a review. Int Biodet Biodeg 46:343-368 [ Links ]
48. Wierzchos J, Ascaso C (1994) Application of back-scattered electron imaging to the study of the lichen rock interface. J Microsc 175:54-59 [ Links ]
49. Wierzchos J, Ascaso C (1998) Mineralogical transformation of bioweathered granitic biotite, studied by HRTEM: evidence for a new pathway in lichen activity. Clays Clay Miner 46:446-452 [ Links ]
50. Wolfaardt GM, Lawrence JR, Korber DR (1999) Function of EPS. In: Wingender J, Neu TR, Flemming H-C (eds) Microbial extracellular polymeric substances. Springer, Berlin, pp 170-200 [ Links ]