My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
International Microbiology
Print version ISSN 1139-6709
INT. MICROBIOL. vol.8 n.4 Dec. 2005
| RESEARCH ARTICLE | |||
|
| |||
| Regulation of conjugal transfer by Lrp and Dam methylation in plasmid R100
Summary. Conjugal transfer of the F-like plasmid R100 occurs at higher frequencies in the absence of DNA adenine methylation. Lower levels of R100-encoded FinP RNA were found in a Dam- host, suggesting that Dam methylation regulates FinP RNA synthesis. Lack of the leucine-responsive regulatory protein (Lrp) causes a decrease in R100 plasmid transfer, indicating that Lrp is an activator of R100-mediated conjugation. Hence, host-encoded regulators previously described for the Salmonella virulence plasmid (pSLT) seem to play analogous roles in R100. Repression of conjugal transfer in rich medium is an additional trait shared by R100 and pSLT. DNA sequence comparisons in regulatory loci support the view that R100 and pSLT are closely related. [Int Microbiol 2005 8(4):279-285] Key words: Dam methylation · fertility inhibition · FinP RNA · leucine-responsive regulatory protein (Lrp) · transcription factor TraJ | ||
| |||
Regulación de la transferencia conjugativa por Lrp y metilación Dam en el plásmido R100 Resumen. La transferencia conjugativa de R100, un plásmido de la familia de F, ocurre a frecuencias más altas en ausencia de metilación Dam. Los mutantes Dam- contienen menores cantidades de RNA FinP que la estirpe silvestre; ello sugiere que la metilación Dam regula la síntesis de RNA FinP. La carencia de proteína reguladora de respuesta a la leucina (Lrp) produce una disminución de la transferencia de R100; ello indica que Lrp es un activador de la conjugación mediada por R100. Por tanto, los reguladores codificados por el hospedador que regulan la transferencia del plásmido de virulencia de Salmonella (pSLT) parecen ejercer funciones análogas en la conjugación mediada por R100. La represión de la transferencia conjugativa en medio rico es otro rasgo común a R100 y pSLT. La comparación de secuencias en loci reguladores apoya la existencia de un estrecho parentesco entre R100 y pSLT. Int Microbiol 2005; 8(4):279-285] Palabras clave: metilación Dam · inhibición de la fertilidad · FinP RNA · proteína reguladora de la respuesta a la leucina (Lrp) · factor transcripcional TraJ | Regulamento da transferência conjugativa por Lrp e metilação Dam no plasmídio R100 Resumo. A transferência conjugativa de R100, um plasmídio da família de F, ocorre a freqüências mais altas em ausência de metilação Dam. Os mutantes Dam- contêm menores quantidades de RNA FinP que a estirpe silvestre; isso sugere que a metilação Dam regula a síntese de RNA FinP. A carência de proteína reguladora da resposta à leucina (Lrp) produz uma diminuição da transferência de R100; isso indica que Lrp é um ativador da conjugação mediada por R100. Portanto, os reguladores codificados pelo hospedador que regulam a transferência do plaásmídio de virulência de Salmonella (pSLT) parecem exercer funções análogas na conjugação mediada por R100. A repressão da transferência conjugativa no meio rico é outro rasgo comum a R100 e pSLT. A comparação de seqüências em loci reguladores apóia a existência de um estreito parentesco entre R100 e pSLT. [Int Microbiol 2005; 8(4):279-285] Palabras chave: metilação Dam · inibição da fertilidade · RNA FinP · proteína reguladora da resposta à leucina (Lrp) · factor transcripcional TraJ |
Introduction
In γ-Proteobacteria, postreplicative methylation of the adenine moiety embedded in 5' GATC 3' sites by the Dam methyltransferase regulates DNA replication, mismatch repair, chromosome segregation, and expression of certain genes [13-15, 17,20]. Dam methylation is also involved in host-pathogen interactions [12,15] and in the regulation of conjugal transfer of the Salmonella virulence plasmid [3] and the F sex factor [26]. In the virulence plasmid of Salmonella enterica serovar Typhimurium (pSLT), expression of the transfer operon (tra), which is tightly repressed in the wild-type, becomes derepressed in a Dam- background [25]. Dam-mediated repression of the tra operon involves two concerted actions: (i) activation of finP transcription [5], which results in high levels of FinP, a small untranslated RNA that prevents traJ mRNA translation [8,9,22]; (ii) repression of traJ transcription [4], which prevents synthesis of TraJ, a transcription factor required for tra operon expression [8,9,22]. Synthesis of the conjugation inhibitor FinP is also activated by Dam methylation in the F sex factor [26]. Both pSLT and F undergo elevated frequencies of conjugal transfer when a Dam- mutant is used as donor [3,26].
Another host-encoded regulator of pSLT plasmid transfer is the leucine-responsive regulatory protein (Lrp), which activates traJ expression [3]. Lrp is a global regulator of the bacterial cell, and can activate or repress gene transcription [2]. In the Salmonella virulence plasmid, Lrp binds to two cognate sites (LRP-1 and LRP-2) in the traJ upstream activating sequence (UAS). Both sites are necessary for Lrp-mediated activation of traJ transcription. Binding of Lrp to the traJ UAS is hindered by methylation of a GATC site located within LRP-2 [4]. Hence, Dam methylation is a transcriptional activator of finP and a transcriptional repressor of traJ. These opposite actions are, however, congruent, since both lead to repression of the transfer operon.
The DNA sequence of the traJ/finP region is highly conserved among F-like plasmids [26], raising the possibility that the host-encoded regulators of conjugation described for pSLT [3-5,26] might be shared by other members of the F-like family. On these grounds, in the present study we investigated whether R100, an antibiotic-resistance plasmid originally found in Shigella [19], was likewise regulated by Lrp and Dam methylation. We found that conjugal transfer of R100 follows rules analogous to those described for the Salmonella virulence plasmid: repression by Dam methylation, activation by Lrp, and inhibition of conjugation in rich medium. In contrast, some such rules are not followed by the F sex factor, indicating that the regulatory design shared by R100 and pSLT is not present in all members of the F-like family.
Materials and methods
Bacterial strains and plasmids. The strains of E. coli and Salmonella enterica used in this study are listed in Table 1. All S. enterica strains belong to serovar Typhimurium, and derive from strain LT2. Strains AB1157, GM28, and GM3819 are Escherichia coli K-12 derivatives provided by M.G. Marinus (Dept. Pharmacology, Univ. of Massachusetts, Worcester, MA, USA). The original source of plasmid R100 was E. coli EC1005/ R100, provided by R. Díaz-Orejas (Biological Research Center, CSIC, Madrid, Spain). Since wild-type R100 is unstable in S. enterica [11,28], a spontaneous R100 derivative that contained the RTF region but lacked the r-determinant region was used. Such R100 derivatives confer tetracycline resistance, and can be stably propagated in S. enterica [11,28]. Plasmid pIZ954 is a pMD1405 derivative carrying an R100 traJ::lac translational fusion. pMD1405 (Apr), obtained from M. Drummond (John Innes Institute, Norwich, England), is a ColE1 derivative engineered for the construction of translational fusions with a lacZ gene that lacks the first eight codons. The lrp-41::Tn5 allele was a gift from D.R. Hillyard (Dept. Pathology, Univ. of Utah, Salt Lake City, UT, USA). The episome F"114 lac+ zzf::Tn10dTc was obtained from J.R. Roth (Section of Microbiology, Univ. of California, Davis, USA).
Culture media and growth conditions. The minimal media were M9 for E. coli [18] and E for S. enterica [27]. The rich medium was nutrient broth (8 g/l, Difco) with added NaCl (5 g/l). Solid media contained agar at 1.5%, final concentration. Auxotrophic supplements and antibiotics were used at the final concentrations described by Maloy [16].
Matings. Aliquots from late-exponential cultures of the donor and the recipient were mixed 1:1, harvested by centrifugation, and washed with minimal medium (M9 for E. coli, E for S. enterica). After washing, the mating mixtures were vacuum-filtered onto the surface of a Millipore membrane filter (0.45-µm pore size); the filter was then placed on an appropriate plate and incubated at 37ºC. After mating, the mixtures were diluted and spread (both diluted and undiluted) on selective agar. As controls, aliquots of the donor and the recipient cultures were also spread separately on selective plates. Conjugation frequencies were calculated per donor bacterium.
β-Galactosidase assays. Levels of b-galactosidase activity were assayed as described by Miller [18], using the CH3Cl-SDS permeabilization procedure.
RNA extraction. RNA preparations were obtained by guanidinium isothiocyanate lysis and phenol:chloroform extraction [6]. Saturated cultures were immersed in liquid N2, and 1.4 ml of lysis solution (5 M guanidinium isothiocyanate, 50 mM Tris (pH 7.5), 10 mM EDTA, 8% (v/v) b-mercaptoethanol) was added. Each mixture was incubated at 60oC for 10 min, before the addition of 0.28 ml of chloroform. After gentle shaking and centrifugation at 9,000 rpm for 10 min, 0.66 ml of isopropanol were added to the supernatant. The samples were incubated at -20°C for 15 min, and centrifuged again at 9,000 rpm for 15 min. The pellets were then rinsed with 70% ethanol, and dried. After resuspension in 75 µl of water (with 0.1% (v/v) diethyl pyrocarbonate, DEP), the samples were subjected to standard treatment with deoxyribonuclease and proteinase K, followed by extraction with phenol:chloroform:isoamyl alcohol, and chloroform:isoamyl alcohol. The aqueous phase was precipitated with 1/10 volume of 3 M sodium acetate (pH 4.8) and 2.5 volumes of absolute ethanol. The samples were then kept at -78°C for at least 30 min, centrifuged, and washed with 70% ethanol. Finally, the precipitates were dried and resuspended in 20-40 µl of DEP-water.
RNA electrophoresis in polyacrylamide gels. Samples consisted of 6 µl of the RNA preparation and 4 µl of loading buffer containing 50% formamide. After a 2-min incubation at 94°C, the samples were chilled in ice. Electrophoretic separation was carried out on gels prepared with TBE and containing 8% acrylamide and 7.5 M urea. The gels were 12-cm long and 0.75-mm thick. Electrophoretic separation was done at 250 V.
RNA hybridization against DNA probes. After electrophoretic separation of RNA, polyacrylamide gels were treated with cold TBE (0.5×) for 15 min. The RNA bands were transferred to nylon filters with a Transblot SD Semidry Transfer Cell system from BioRad Lab. (Richmond, CA, USA). Transfer was allowed to proceed for 1 h at 400 mA, at intensities below 25 V. After transfer, the filters were stained with a solution of 0.3% methylene blue in 0.3 M sodium acetate (pH 5.2) to confirm both the efficiency of transfer and the presence of equivalent amounts of RNA per lane. As a loading control, ribosomal RNA fragments, readily visible in the stained gel, were quantified using the Image Gauge software in a Fujifilm FLA-3000 Image System. The presence of equivalent amounts of RNA per lane was thus confirmed. Loads that showed differences below 10% were not considered significant. The probe used for Northern hybridization experiments was 5' TAA ATC GCC GAT ACA GGG AG 3'. This sequence is complementary to the 3' end of F-encoded FinP RNA and is 90% homologous (2 nucleotide changes) to the corresponding region of R100 (EMBL accession numbers M13054 and U01159). The probe was end-labelled with [γ32P]ATP. Prehybridization and hybridization were carried out at 38°C, and formamide was not used. After hybridization, the nylon filters were washed twice at room temperature with 6× SSC, 0.1% SDS during 5 min. Two or more additional washes were carried out with either 0.6× SSC, 0.1% SDS or 3× SSC, 0.1% SDS during 15 min at room temperature. The filters were then exposed to an X-ray film for 1-7 days.
Results
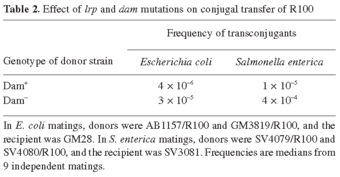
Conjugal transfer of R100 from Dam+ and Dam- donors. Aliquots of 0.5 ml from late-exponential cultures of the donor (E. coli AB1157/R100 or E. coli GM3819/R100) and the recipient (E. coli GM28) were mixed, pelleted by centrifugation, and resuspended in 0.02 ml of M9 buffer. The mating mixtures were then vacuum-transferred to a filter (pore size: 0.45 µm), placed on an LB plate, and incubated for 90 min at 37°C. To interrupt mating, the mixtures were resuspended in M9 buffer, vigorously shaken, and diluted ten-fold before plating on M9 supplemented with tetracycline. The presence of a dam mutation in the donor increased R100 transfer around ten-fold (Table 2). Hence, transfer of R100 between E. coli strains is repressed by Dam methylation, as previously described for F [26] and for the Salmonella virulence plasmid [3].
The effect of a dam mutation on R100 transfer was also examined in S. enterica serovar Typhimurium, using strains SV4079/R100 and SV4080/R100 as donors and SV3081 as the recipient. For these experiments, a Tcr R100 derivative lacking the r-determinant region was used (see Materials and methods). To avoid potential cross-regulation by the FinO and FinP products of the Salmonella virulence plasmid, the experiments were carried out in the absence of pSLT. Matings were carried out as above, except that transconjugants were selected on E plates with tetracycline. The presence of a dam mutation in the donor increased R100 transfer 50-fold (Table 2). This difference is larger than that found between Dam+ and Dam- donors of E. coli and may have two causes: (i) the smaller R100 derivative may be less repressed than the parent plasmid; (ii) repression of conjugation may be tighter in E. coli K-12 than in S. enterica LT2 (data not shown).
Synthesis of R100-encoded FinP RNA in Dam+ and Dam- hosts. A previous study had shown that Dam- mutants of S. enterica contained lowered levels of FinP RNA encoded by the virulence plasmid [26]. Reduced amounts of F-encoded FinP RNA were likewise found in Dam- mutants of E. coli [26]. Based on these antecedents, we investigated the effect of Dam methylation on the synthesis of R100-encoded FinP RNA in E. coli AB1157/R100 (Dam+) and GM3819/R100 (Dam-). Total RNA was prepared from saturated cultures of both strains, which were grown in LB. Northern hybridization experiments showed that FinP RNA was more abundant in the Dam+ host (Fig. 1A). Densitometric analysis indicated that the difference in FinP RNA content between Dam+ and Dam- hosts was around three-fold (data not shown). Hence, the FinP RNA shortage can be postulated as a likely cause of R100 conjugal derepression in a Dam- host. A side observation was that the difference in FinP RNA content between Dam+ and Dam- hosts was smaller than the difference in conjugation frequencies described in the former section. This observation can be explained by a cascade effect: a small difference in the level of FinP RNA can cause a larger increase in TraJ translation, which can be further amplified at the level of tra operon expression [8]. An alternative explanation may be that Dam methylation regulates also traJ transcription, as described in the Salmonella virulence plasmid [4]. The two explanations are not mutually exclusive.
Expression of the R100 traJ gene in Dam+ and Dam- hosts. Lowered levels of FinP RNA can be expected to increase the stability of traJ mRNA; in turn, increased synthesis of the TraJ transcription factor can be expected to derepress the tra operon. To test this hypothesis, the activity of a translational traJ::lac fusion was compared in Dam+ and Dam- hosts (AB1157 and GM3819, respectively). Since plasmid pIZ954 carries a translational fusion traJ::lac as well as the finP promoter and the complete finP gene, FinP shortage should result in increased expression of the traJ::lac translational fusion. Derepression of TraJ translation in a Dam- background was indeed observed (Fig, 1B), thereby providing further evidence that the absence of DNA adenine methylation causes FinP scarcity.
Conjugal transfer of R100 from Lrp+ and Lrp- donors. The involvement of Lrp in transcriptional activation of the traJ gene in the Salmonella virulence plasmid [3,4] raised the possibility that a similar control might operate in R100. Hence, matings were performed to compare R100 plasmid transfer from isogenic Lrp+ and Lrp- donors of S. enterica (SV4419/R100 and SV4302/R100, respectively). The recipient was SV3081, a pSLT-cured strain. Transconjugants were selected on minimal E plates supplemented with tetracycline; use of minimal medium counterselected the auxotrophic donors. A ten-fold decrease in the frequency of R100 transfer was observed when an Lrp- donor was used (Table 3). This difference provided evidence that that Lrp acts as a conjugation activator in plasmid R100.
R100 transfer from Dam- Lrp+ and Dam- Lrp- donors (SV4303/R100 and SV4306/R100, respectively) was also examined. A dam mutation increased conjugal transfer 50-fold, and the presence of both dam and lrp mutations in the donor strain restored the repression of R100 transfer to a level similar to that of the wild-type (Table 3). These observations confirmed that dam and lrp mutations exert opposite effects in the regulation of R100 transfer, as previously described for the Salmonella virulence plasmid [3,4,26].
Since pSLT transfer is known to be repressed in rich media [1], conjugal transfer of R100 was also assayed in LB. The donor strains for these experiments were R100-carrying derivatives of SV3081 (Dam+ Lrp+), SV3082 (Dam- Lrp+), SV4714 (Dam+ Lrp-), and SV4715 (Dam- Lrp-), and the recipient was SV3150. Transconjugants were selected on LB plates supplemented with tetracycline and chloramphenicol. Tight repression of conjugal transfer was observed in all donor backgrounds, and the presence of dam and lrp mutations, alone or combined, in the donor strain caused minor effects (Table 3). Hence, R100 transfer under such conditions is repressed by a mechanism independent from both Dam methylation and Lrp. In pSLT, inhibition of conjugal transfer in rich medium seems to be caused by repression of traJ transcription (A. Serna, unpublished data). Given the regulatory analogies between both plasmids, we tentatively postulate an analogous mechanism for R100.
Role of Lrp in conjugal transfer of other F-like plasmids: comparison between R100 and F. Repression of conjugal transfer in rich medium, a trait shared by R100 and pSLT, is not observed in the F sex factor [8]. Another difference is that F transfer is repressed in stationary cultures [10], while transfer of pSLT is not [5]. These observations suggest that individual regulatory differences exist among F-like plasmids. On these grounds, we examined whether Lrp-mediated activation of conjugal transfer, a mechanism shared by pSLT and R100, occurred also in the F sex factor. For this purpose, two couples of isogenic Lrp+ and Lrp- Salmonella strains, each containing either F"114 lac+ zzf::Tn10dTc or R100 Tcr, were used as donors in mating experiments. The recipient strain was SV3081. Matings were performed in E medium, and transconjugants were selected on E plates supplemented with tetracycline. Unlike R100, F"114 lac+ zzf::Tn10dCm was found to be insensitive to the presence of an lrp mutation (Table 4), suggesting that Lrp is not an activator of conjugal transfer in the F sex factor. Another F-like plasmid showing Lrp-insensitive conjugal transfer has been previously described [24].
Discussion
Like pSLT and other F-like plasmids that carry a complete FinOP system of fertility inhibition [8], R100 is transferred at low frequencies in wild-type E. coli and Salmonella. Derepression of R100 transfer in a Dam- donor can be correlated with the existence of lowered levels of FinP RNA, as previously described for F and pSLT [26]. In the latter, Dam methylation has been shown to prevent HN-S mediated repression of the finP promoter, a phenomenon that remains to be deciphered at the molecular level [5]. The high conservation of DNA sequence in the R100 and pSLT finP promoter regions (Fig. 2) suggests that an analogous mechanism operates in R100
An additional trait shared by pSLT and R100 is activation of conjugal transfer by Lrp. Again, sequence conservation in the upstream-activating-sequence of the traJ gene (Fig. 3) suggests that Lrp exerts analogous functions in R100 and pSLT. In the latter, Lrp binds to two cognate sites upstream from the traJ promoter [4]. Transcriptional activation requires Lrp binding to both sites (LRP-1 and LRP-2). Methylation of a GATC site present in LRP-2 hinders Lrp binding and represses traJ transcription [4]. In a Dam- mutant, increased binding of Lrp to the traJ UAS leads to transcriptional activation of the traJ gene [4]. Hence, derepression of pSLT conjugal transfer in a Dam- background can be attributed to both decreased finP transcription and increased traJ trancription [3,4]. One of the putative Lrp-binding sites found in the traJ UAS of R100 (LRP-1) shows more differences (6/15 plus one insertion) with the consensus Lrp-binding sequence than pSLT (3/15 plus one insertion). In contrast, 9/15 nucleotides match the consensus in the LRP-2 site of both pSLT and R100 (Fig. 2). As Lrp is a rather promiscuous DNA-binding protein [2], inference from analysis in silico must be cautious. However, the existence of close-to-consensus LRP-1 and LRP-2 sites strongly suggests that Lrp-mediated activation of traJ expression occurs in R100. The additional observation that LRP-2 contains a GATC motif may also provide evidence that transcription of the R100 traJ gene is regulated by Dam methylation, as described for pSLT [4].
The analogies found between R100 and pSLT do not fully extend, however, to the F sex factor. Conjugal transfer of the F sex factor is repressed by Dam methylation [26], but it is not activated by Lrp. Both the analogy and the difference may be reflected in nucleotide sequences: while the finP promoter is highly conserved in F, pSLT, and R100 (Fig. 2), the putative LRP-2 site the traJ UAS of F shows significant divergence with both the consensus and the LRP-2 regions of pSLT and R100 (Fig. 3). Furthermore, the GATC site that represses Lrp binding to the traJ UAS in pSLT is not found in F, thereby ruling out the possibility that Dam methylation regulates traJ transcription in the F sex factor.
The view that F-like plasmids share a general regulatory design compatible with plasmid-specific regulatory traits is supported by additional lines of evidence: (i) CRP is an activator of conjugal transfer in pRK100 [23] but not in pSLT (A. Serna, unpublished); (ii) the nucleoid protein H-NS, which acts as a repressor of conjugal transfer in F upon entry into stationary phase [29], is an activator of mating in plasmid pRK100 [24]; (iii) transfer of the Salmonella virulence plasmid is repressed in rich medium [1] but this is not true for F sex factor [8]; and (iv) the sudden decrease in F plasmid transfer upon entry into stationary phase [10] is not observed in pSLT [5]. The significance of these differences remains to be established. An appealing hypothesis is that conjugal transfer responds to physiological or environmental signals, and that each plasmid evolved a distinct conjugation strategy.
Acknowledgments. This study was supported by the Spanish Ministry of Education and Science and the European Regional Fund (grant BIO2004-03455-CO2-02). Strains were kindly provided by Ramón Díaz-Orejas, Martin Drummond, David Hillyard, Martin Marinus, and John Roth. We thank Werner Arber, David Low, Silvia Marqués, and Joaquín Torreblanca for helpful discussions.
References
1. Ahmer BMM, Tran M, Heffron F (1999) The virulence plasmid of Salmonella typhimurium is self-transmissible. J Bacteriol 181:1364-1368 [ Links ]
2. Calvo JM, Matthews RG (1994) The leucine-responsive regulatory protein, a global regulator of metabolism in Escherichia coli. Microbiol Rev 58:466-490 [ Links ]
3. Camacho EM, Casadesús J (2002) Conjugal transfer of the virulence plasmid of Salmonella enterica is regulated by the leucine-responsive regulatory protein and DNA adenine methylation. Mol Microbiol 44:1589-1598 [ Links ]
4. Camacho EM, Casadesús J (2005) Regulation of traJ transcription in the Salmonella virulence plasmid by strand-specific DNA adenine hemimethylation. Mol Microbiol 57:1700-1718 [ Links ]
5. Camacho EM, Serna A, Madrid C, Marqués S, Fernández R, de la Cruz F, Juárez A, Casadesús J (2005) Regulation of finP transcription by DNA adenine methylation in the virulence plasmid of Salmonella enterica. J Bacteriol 187:5691-5699 [ Links ]
6. Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidine thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156-159 [ Links ]
7. Fee BE, Dempsey WB (1986) Cloning, mapping and sequencing of plasmid R100 traM and finP genes. J Bacteriol 167:336-345 [ Links ]
8. Firth N, Ippen-Ihler K, Skurray RA (1996) Structure and function of the F factor and mechanism of conjugation. In: Neidhardt FC, Curtiss R III, Ingraham JL, Lin ECC, Low KB, Magasanik B, Reznikoff WS, Riley M, Schaechter M, Umbarger HE (eds) Escherichia coli and Salmonella. Cellular and molecular biology. ASM Press, Washington, DC, pp. 2377-2401 [ Links ]
9. Frost LS, Ippen-Ihler K, Skurray RA (1994) Analysis of the sequence and gene products of the transfer region of the F sex factor. Microbiol Rev 58:162-210 [ Links ]
10. Frost LS, Manchak J (1998) F-phenocopies: characterization of expression of the F transfer region in stationary phase. Microbiology 144:2579-2587 [ Links ]
11. Gómez-Eichelmann MC, Torres HK (1983) Stability of plasmids R1-19 and R100 in hyper-recombinant Escherichia coli strains and in Salmonella typhimurium strains. J Bacteriol 154:1493-1497 [ Links ]
12. Hernday A, Krabbe M, Braaten B, Low D (2002) Self-perpetuating epigenetic pili switches in bacteria. Proc Natl Acad Sci USA 99S4:16470-16476 [ Links ]
13. Løbner-Olesen A, Marinus MG, Hansen FG (2003) Role of SeqA and Dam in Escherichia coli gene expression: a global/microarray analysis. Proc Natl Acad Sci USA 100:4672-4677 [ Links ]
14. Løbner-Olesen A, Skovgaard O, Marinus MG (2005) Dam methylation: coordinating cellular processes. Curr Op Microbiol 8:154-160 [ Links ]
15. Low DA, Weyand NJ, Mahan MJ (2001) Roles of DNA adenine methylation in regulating bacterial gene expression and virulence. Infect Immun 69:7197-7204 [ Links ]
16. Maloy SR (1990) Experimental techniques in bacterial genetics. Jones and Bartlett Publishers, Boston [ Links ]
17. Marinus MG (1996) Methylation of DNA. In: Neidhardt FC, Curtiss III R, Ingraham JL, Lin ACC, Low KB, Magasanik B, Reznikoff WS, Riley M, Schaechter M, Umbarger HE (eds) Escherichia coli and Salmonella. Cellular and molecular biology. ASM Press, Washington, DC, pp. 782-791 [ Links ]
18. Miller JH (1992) A short course in bacterial genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [ Links ]
19. Nakaya R, Nakamura A, Murata Y (1960) Resistance transfer agents in Shigella. Biochim Biophys Res Commun 3:654-659 [ Links ]
20. Oshima T, Wada C, Kawagoe Y, Ara T, Maeda M, Masuda Y, Hiraga S, Mori H (2002) Genome-wide analysis of deoxyadenosine methyltransferase-mediated control of gene expression in Escherichia coli. Mol Microbiol 45:673-695 [ Links ]
21. Penfold SS, Simon J, Frost LS (1996) Regulation of the expression of the traM gene of the F sex factor of Escherichia coli. Mol Microbiol 20:549-558 [ Links ]
22. Rotger R, Casadesús, J. (1999) The virulence plasmids of Salmonella. Int Microbiol 2: 177-184 [ Links ]
23. Starcic M, Zgur-Bertok D, Jordi BJAM, Wösten MMSM, Gaastra W, van Putten JPM (2003) The cyclic AMP-cyclic AMP receptor protein complex regulates activity of the traJ promoter of the Escherichia coli conjugative plasmid pRK100. J Bacteriol 185:1616-1623 [ Links ]
24. Starcic-Erjavec M, van Putten JP, Gaastra W, Jordi BJ, Grabnar M, Zgur-Bertok D (2003) H-NS and Lrp serve as positive modulators of traJ expression from the Escherichia coli plasmid pRK100. Mol Genet Genomics 270:94-102 [ Links ]
25. Torreblanca J, Casadesús J (1996) DNA adenine methylase mutants of Salmonella typhimurium and a novel Dam-regulated locus. Genetics 144:15-26 [ Links ]
26. Torreblanca J, Marqués S, Casadesús J (1999) Synthesis of FinP RNA by plasmids F and pSLT is regulated by DNA adenine methylation. Genetics 152:31-45 [ Links ]
27. Vogel HJ, Bonner DM (1956) Acetylornithase of Escherichia coli: partial purification and some properties. J Biol Chem 218:97-106 [ Links ]
28. Watanabe H, Katsutoshi M, Hashimoto H (1982) Recombination between two IS1s flaking the r-determinant of R100-1: involvement of dor and recA gene functions in Salmonella typhimurium. J Bacteriol 150:113-121 [ Links ]
29. Will WR, Lu J, Frost LS (2004) The role of H-NS in silencing F transfer gene expression during entry into stationary phase. Mol Microbiol 54:769-782 [ Links ]