Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
International Microbiology
versão impressa ISSN 1139-6709
INT. MICROBIOL. vol.9 no.1 Mar. 2006
Light-induced rhythmic changes in thermotolerance in stationary-phase cells of Candida utilis
Miguel A. Lapeña; Jero Vicente-Soler; Teresa Soto; Marisa Madrid; Andrés Núñez;
Encarnación García; José Cansado; Mariano Gacto
Department of Genetics and Microbiology, Faculty of Biology, University of Murcia, Spain
SUMMARY
In synchronized light-dark cycles, stationary-phase cultures of the budding yeast Candida utilis were able to survive heat treatment at 50ºC with an apparent circadian-like rhythm related to the onset of light. However, in continuous darkness this pattern did not run freely and was markedly dampened. We discuss these findings in terms of the potential circadian control of heat tolerance, which has been described in the fission yeast Schizosaccharomyces pombe. Our results suggest that the resistance pattern observed in C. utilis is most likely an adaptive response to the light-induced generation of reactive oxygen species rather than the occurrence of a truly endogenous circadian rhythm. [Int Microbiol 2006; 9(1):61-64].
Key words: Candida utilis · thermotolerance · circadian rhythms · light
RESUMEN
Cambios rítmicos inducidos por la luz en la termotolerancia en la fase estacionaria de Candida utilis
La supervivencia de cultivos de Candida utilis en la fase estacionaria tras ser sometidos a temperaturas de 50ºC en ciclos sincronizados de luz/oscuridad presentó un aparente ritmo circadiano relacionado con el inicio de la fase iluminada. Sin embargo, en condiciones de oscuridad continua este patrón no se observaba tan claramente y mostraba una marcada ambigüedad. Estas observaciones se discuten en términos de un posible control circadiano de la tolerancia a altas temperaturas, que ha sido descrito para la levadura Schizosaccharomyces pombe. Nuestros resultados indican que el patrón de resistencia observado en C. utilis es muy probablemente una respuesta adaptativa a la generación de especies reactivas de oxígeno inducida por luz y que no existe un verdadero ritmo circadiano endógeno. [Int Microbiol 2006; 9(1):61-64].
Palabras clave: Candida utilis · termotolerancia · ritmos circadianos · luz
RESUMO
Mudanças rítmicas induzidas pela luz na termotolerância na fase estacionária de Candida utilis
A sobrevivência de cultivos de Candida utilis na fase estacionária depois de ser submetidos a temperaturas de 50ºC em ciclos sincronizados de luz/escuridão apresentou um aparente ritmo circadiano relacionado com o início da fase iluminada. No entanto, em condições de escuridão contínua este patrão não foi observado tão claramente e mostrou uma marcada ambigüidade. Estas observações são discutidas com relação a um possível controle circadiano da tolerância a altas temperaturas, que foi descrito para a levedura Schizosaccharomyces pombe. Nossos resultados indicam que o patrão de resistência observado em C. utilis é muito provavelmente uma resposta adaptativa à geração de espécies reativas de oxigênio induzida por luz e que não existe um verdadeiro ritmo circadiano endógeno. [Int Microbiol 2006; 9(1):61-64].
Palavras chave: Candida utilis · termotolerância · ritmos circadianos · luz
Introduction
Circadian rhythms represent a biological adaptation to daily fluctuations in the environment [4], for example, the alternation of light and darkness. This change is important in nature because light normally precedes temperature upshifts, which may act as a physiological challenge to organisms. There are various reports in the literature on the presence of rhythms of heat tolerance in animals and in plants [6,8,12-14]. In the search for a "circadian minimal system", several chronobiological studies have focused on the fission yeast Schizosaccharomyces pombe. The results appear to support the existence in this yeast of a true circadian rhythm of heat tolerance [9-11].
Reactive oxygen species (ROS) are invariably produced in aerobic environments through a variety of mechanisms, such as electron "leakage" during biological oxidations, the action of flavin dehydrogenases, and direct physical activation of oxygen molecules by radiation energy [1,2]. ROS include superoxide anion, hydrogen peroxide, and hydroxyl radicals, all of which are highly reactive and affect many types of cells by inducing an intracellular oxidative stress [2]. Although speculation remains regarding the precise mechanisms involved, irradiation of cells by light greatly increases the generation of ROS, indicating that ROS formation is light-sensitive [3]. In fact, ROS produced during light processes have a role at the cellular level in the etiology of many light-induced disorders [3]. Intracellular production of ROS is also of fundamental importance for the triggering of evolutionarily conserved mitogen-activated-protein kinase (MAPK) signaling pathways, which regulate the response to many environmental stresses [7]. The downstream effect of pathway activation is the increased transcription of genes involved in the detoxification of ROS and of genes collectively involved in the "general stress response"; this, in turn, regulates the adaptive response of cells to external stimuli, including thermotolerance [16]. It has also been shown that ROS inhibition of tyrosine-phosphatases that dephosphorylate effector kinases of the MAPK pathways may help the propagation and maintenance of MAPK signals mediated by protein phosphorylation [5].
To our knowledge, studies linking light and thermotolerance similar to those performed in the fission yeast S. pombe [9-11] are lacking in budding yeast. Hence, our results provide the first report of a similar response in the budding yeast Candida utilis, which is phylogenetically distant from fission yeast [15]. However, our interpretation of the observations made in C. utilis differs from that in previous studies in which S. pombe was used as the model organism.
Materials and methods
Organism and culture conditions
The yeast strain Candida utilis CECT1061, an anamorphic form of Pichia jadinii, was used in this study. Liquid cultures were grown until stationary phase at 28ºC on a rotary shaker in YEPD medium containing 2% glucose (as carbon source), 2% peptone, and 4% yeast extract (w/v). Cells were seeded and subsequently maintained under 12:12 h light-dark cycles for at least 4 days before the tests were done. The cycles consisted of about 3000 lx white fluorescent light during the light phase (Osram L18W/20A Coldwhite; 12.3 W/m2) and no light during the dark phase. Aliquots for treatments were withdrawn and appropriately diluted into YEPD medium prewarmed at 45 or 50ºC. After heat exposure for 20 min or the specified time intervals, the samples were vortexed and plated in triplicate at room temperature. Following 4 days of incubation at 28ºC, the colonies were counted and the number of surviving cells per ml determined.
Resistance to heat shock treatments
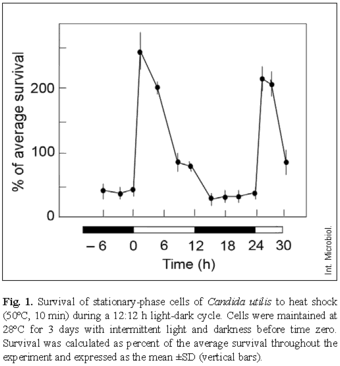
Survival fractions were calculated separately for each culture as percentage with respect to the average of three control platings at different times of the cycle. The data presented in Figs. 1 and 2 were calculated as percent of the average survival, as described in previous reports [9]. Briefly, the mean of all surviving fractions obtained for any individual culture during the experiment was determined and set to 100%. The values for single time points were then expressed as a percentage of this mean value. In experiments of the type shown in Fig. 3, in which dose-response curves were generated for heat treatments of different duration, control platings were done at all heat-shock times and the cell number of the controls was averaged. In all cases, each point represents the mean of three independent measurements.
Results and Discussion
Cells from cultures subjected to several cycles of light and darkness were examined for differences in survival following heat shock (50ºC) at 3-h intervals during a complete 12:12 h light-dark cycle. A typical result is shown in Fig. 1. The data indicate clear fluctuations in heat sensitivity as a function of the light period. The highest resistance to heat consistently occurred early in the light period; however, once triggered by light, resistance was not maintained for the entire illumination interval. Since, in nature, the time of the onset of resistance would correspond to a time when the daily temperature had not become maximal, the adaptive significance of this response remains questionable. Moreover, the highest resistance was transient and did not extend into the light period, when the temperature rose in the continued presence of light, analogous to the situation encountered in nature.
We also analyzed whether the rhythmic change in heat-stress resistance during light:dark cycles was truly endogenous, i.e., due to a circadian rhythm, by observing its continuity in a free-running period of constant darkness. Notably, the response was dampened under these conditions and did not persist under conditions of continuous darkness in the absence of light as stimulus (Fig. 2). Hence, these results show that changes in thermotolerance are not regulated by an endogenous circadian pattern.
Since maximum sensitivity and maximum resistance showed a certain relationship to the onset of light in a light-dark cycle, a dose-response curves was plotted 3 h before and after this time point for both exponentially growing and stationary-phase cultures maintained at 28ºC. For these experiments, two different challenge temperatures (45 and 50ºC) were selected for each growth condition, to account for the fact that the thermotolerance of stationary-phase yeast cells is higher than that of exponentially growing cells. As Fig. 3 shows, the results are congruent with the observations described above. In all cases, cells from either exponential or stationary-phase consistently showed a comparative higher resistance to heat shock when assayed 3 h after, as opposed to 3 h before, the beginning the light phase.
In conclusion, our observations in C. utilis are similar to those that have been described in S. pombe. However, our interpretation of these findings in budding yeast is quite different from that suggested for the fission yeast. While the latter there possesses an endogenous circadian rhythm, true circadian control could not be demonstrated in the budding yeast-in which the light-induced rhythmic changes in thermotolerance were lost when the cells were maintained in darkness. Thus, in C. utilis, it appears that light intensity, rather than having a direct effect by setting in motion a circadian clock, most likely induces the generation of ROS, which, in turn, promote activation of MAPK signaling pathways. In support of this view, there is ample evidence that ROS are generated by light [3], that they are able to transiently activate the general stress response underlying the adaptive transcription of selected genes through MAPK signaling pathways [7], and that the induced genes are involved in both ROS detoxification and the transient development of resistance to various stresses, including thermotolerance [16].
Acknowledgements
M. Madrid received a scholarship from the FPU program of the Ministerio de Educación, Cultura y Deporte, Spain. This work was supported in part by grants BFU2005-01401/BMC from MEC and 00475/PI/04 from Fundación Séneca, Murcia, Spain.
References
1. Andreyev AY, Kushnareva YE, Starkov AA (2005) Mitochondrial metabolism of reactive oxygen species. Biochemistry 70:200-214 [ Links ]
2. Bergamini CM, Gambetti S, Dondi A, Cervelatti C (2004) Oxygen, reactive oxygen species and tissue damage. Curr Pharm Des 10:1611-1626 [ Links ]
3. Black HS (2004) ROS: a step closer to elucidating their role in the etiology of light-induced skin disorders. J Invest Dermatol 122:13-14 [ Links ]
4. Büning E (1973) The physiological clock. Springer, Berlin [ Links ]
5. Chiarugi P (2003) Reactive oxygen species as mediators of cell adhesion. Ital J Biochem 52:28-32 [ Links ]
6. Doi M, Nakajima Y, Okano T, Fukada Y (2002) Light-dependent changes in the chick pineal temperature and the expression of the cHsp90 gene: a potential contribution of in vivo temperature change to the photic-entrainment of the chick pineal circadian clock. Zool Sci 19:633-641 [ Links ]
7. Gacto M, Soto T, Vicente-Soler J, Villa TG, Cansado J (2003) Learning from yeasts: intracellular sensing of stress conditions. Int Microbiol 6:211-219 [ Links ]
8. Hutchison VH, Maness JD (1979) The role of behaviour in temperature acclimation and tolerance in ectotherms. Am Zool 19:367-384 [ Links ]
9. Kippert F (1989) Circadian control of heat tolerance in stationary phase cultures of Schizosaccharomyces pombe. Arch Microbiol 151:177-179 [ Links ]
10. Kippert F (2001) Cellular signaling and the complexity of biological timing: insights from the ultradian clock of Schizosaccharomyces pombe. Philos Trans R Soc London B Biol Sci 356:1725-1733 [ Links ]
11. Kippert F, Lloyd D (1995) A temperature-compensated ultradian clock ticks in Schizosaccharomyces pombe. Microbiology 41:883-890 [ Links ]
12. Li QB, Guy CL (2001) Evidence for non-circadian light/dark-regulated expression of Hsp70s in spinach leaves. Plant Physiol 125:1633-1642 [ Links ]
13. Rensing L, Monnerjahn C (1996) Heat shock proteins and circadian rhythms. Chronobiol Int 13:239-250 [ Links ]
14. Rikin A (1992) Circadian rhythm of heat resistance in cotton seedlings: synthesis of heat shock proteins. Eur J Cell Biol 59:160-165 [ Links ]
15. Russell P, Nurse P (1986) Schizosaccharomyces pombe and Saccharomyces cerevisiae: a look at yeasts divided. Cell 45:781-782 [ Links ]
16. Soto T, Beltran FF, Paredes V, Madrid M, Millar JBA, Vicente-Soler J, Cansado J, Gacto M (2002) Cold induces stress-activated protein kinase-mediated response in the fission yeast Schizosaccharomyces pombe. Eur J Biochem 269:5056-5065 [ Links ]
![]() Corresponding author:
Corresponding author:
M. Gacto
Department of Genetics and Microbiology
Faculty of Biology
University of Murcia
30071 Murcia, Spain
Tel. +34-968367132. Fax +34-968363963
E-mail: maga@um.es
Received 14 November 2005
Accepted 12 December 2005