My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Española de Sanidad Penitenciaria
On-line version ISSN 2013-6463Print version ISSN 1575-0620
Rev. esp. sanid. penit. vol.18 n.3 Barcelona 2016
Pharmacological management of dyslipidemia in high and very high cardiovascular risk patients
Tratamiento farmacológico de la dislipemia en pacientes de alto y muy alto riesgo cardiovascular
V. Pascual Fuster
Coordinator of the Primary Healthcare Group of the Spanish Arteriosclerosis Society.
ABSTRACT
Dyslipaemia is one of the main risk factors in the development of cardiovascular diseases. Currently, there are different alternatives available (amongst which statins occupy a pre-eminent place), to optimise the treatment of patients at high or very high cardiovascular risk.
Despite this, the percentage of patients that achieve good lipid control is low. The causes of the mismatch with proposed objectives include lack of patient adherence and therapeutic inertia.
This review uses available evidence and the latest clinical guides as a basis to assess the pharmacological treatment of dyslipaemia in patients with a background of arteriosclerotic vascular disease, diabetes, chronic kidney disease, cardiovascular risk at ≥5% calculated by SCORE and familial hypercholesterolaemia.
The treatment of hypertriglyceridemia is also reviewed along with the special consideration that poly-pharmacy deserves in patients treated with statins, making mention of the treatment of dyslipaemia with HIV infection.
The global assessment of cardiovascular risk is of high priority to adapt treatment to the specific objectives of the c-LDL for each risk category.
Keywords: prisons; hydroxymethylglutaryl-CoA reductase inhibitors; risk factors; cardiovascular diseases; hypolipidemic agents; primary prevention; HIV; Spain.
RESUMEN
La dislipemia es uno de los principales factores de riesgo para el desarrollo de enfermedades cardiovasculares. Actualmente disponemos de diferentes alternativas (entre las que las estatinas ocupan un lugar preeminente), para optimizar el tratamiento en los pacientes de alto y muy alto riesgo cardiovascular.
A pesar de ello, el porcentaje de pacientes que consigue un buen control lipidico es bajo, entre las causas de la inadecuacion a los objetivos propuestos, figuran: la falta de adherencia del paciente y la inercia terapeutica.
En esta revision se valora en base a las evidencias disponibles y a las ultimas guias clinicas, el tratamiento farmacologico de la dislipemia en pacientes con antecedentes de enfermedad vascular arteriosclerotica, diabetes, enfermedad renal cronica, riesgo cardiovascular .5% calculado por SCORE e hipercolesterolemia familiar.
Tambien se revisa el tratamiento de la hipertrigliceridemia y la consideracion especial que merece la polimedicacion en pacientes tratados con estatinas, haciendo mencion al tratamiento de la dislipemia en aquellos con infeccion por VIH.
La evaluacion global del riesgo cardiovascular es prioritaria para adecuar el tratamiento a los objetivos especificos del c-LDL para cada categoria de riesgo.
Palabras clave: prisiones; estatinas HMG-CoA; factores de riesgo; enfermedades cardiovasculares; hipolipemiantes; prevencion primaria; VIH; Espana.
Introduction
Dyslipidemia is defined as increased plasma concentrations of cholesterol and/or triglycerides, or an isolated reduction of high-density lipoprotein cholesterol (c-HDL).
The increase of cardiovascular risk derived from elevated plasma cholesterol is progressive and continuous for concentrations over 200mg/dl. Low-density lipoprotein cholesterol (LDL-c) plays an atherogenic role while c-HDL acts as a protective factor.
Dyslipidemia is one of the mains risk factors for coronary artery disease (CAD) and cerebrovascular disease, correct diagnosis and appropriate treatment are therefore essential in the prevention of cardiovascular disease (CVD). Even though, the rate of patients that achieve appropriate lipid control, especially high and very high cardiovascular risk patients, is very low1. CLINICOR2 trial, which included 1137 moderate to very high cardiovascular risk patients, concluded that only 9% gained control over LDL-c concentrations.
However, the view of clinicians is different since control of dyslipidemia is frequently overestimated, as the LIPEDIA3 study concludes, where most of the clinicians interviewed regarding the treatment and monitoring of diabetic patients considered that over 50% achieve pre-established lipid-lowering targets. Data from the Hispalipid study show that clinicians estimate that 40% of their high risk patients have target LDL-c figures, while when reviewing actual data only 15% did4. A recently published study5 goes along the same lines of misperception of the clinical reality and it gathers the opinion of both primary care clinicians and cardiologists regarding patient followup after acute coronary syndrome (ACS). This same study5 takes into account the lack of treatment adherence as the leading cause for not meeting the targets over clinical inertia. However existing data suggests that in high risk patients there is therapeutic inertia in 70% of all cases6 a relevant issue since such inertia and consequent lack of intensive treatment, is associated to a clearly increased risk of ischemic events.
Therefore, the treatment of dyslipidemia is essential in reducing cardiovascular morbimortality particularly in high and very high cardiovascular risk patients and clinicians show an optimistic view of the degree of lipid control of their patients. Thus the implementation of measures to improve the treatment of dyslipidemia and adapt it to the targets recommended by clinical practice guidelines remains a priority. The latest European guidelines on cardiovascular prevention7, including the consensus document of the Task Force for the management of dyslipidemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS) which establishes several levels of overall cardiovascular risk with their corresponding LDL-c control objective, as the recently published "Consensus document of the Medical Associations in the Autonomous Community of Valencia for the clinical-practical management of dyslipidemia" that also includes the recommended treatment for each risk category9 (see Table 1).
We will now specify the main features of dyslipidemia treatment for different types of patients:
1. Patients with known atherosclerotic vascular disease
European guidelines published in 20118 consider that patients with a record of myocardial infarction (MI), acute coronary syndrome (ACS), coronary artery bypass or other arterial revascularization procedure, ischemic stroke, peripheral artery disease (PAD) or CVD evidenced by any invasive or non-invasive test are very high cardiovascular risk patients and establish a LDL-c target of less than 70mg/dl or a reduction of basal levels of 50%8. They also recommend lifestyle modifications including increased physical activity, weight control and quit smoking. Moreover, treatment should be initiated with the most appropriate status according to its efficacy and basal LDL-c and if not contraindicated, at high doses. Furthermore, patients with triglyceridemia >200mg/dl require non-HDL cholesterol control of <100mg/dl8.
Statins reduce the synthesis of cholesterol in the liver by inhibiting the activity of HMG-CoA reductase leading to reduced plasma cholesterol. The CTT meta-analysis on data from over 170000 participants of 26 randomized clinical trials10, established that statin treatment reduced cardiovascular mortality in 20% per every 1.0mmol/l (39mg/dl) reduction of LDL-c. These results are indicative of the fact that clinical benefit depends on the degree of LDL-c reduction. Therefore, the election of statin should reflect the degree of LDL-c reduction required to achieve the target concentration for a particular patient (Table 2)11-13. The statins for which a reduction of LDL-c of over 50% on maximum doses are expected are atorvastatin (80mg/dl) and rosuvastatin (20 mg/dl)8,14.
The benefit of intensive treatment with statins has been stated in clinical trials such as TNT15 which compares two lipid-lowering strategies in patients with CAD: atorvastatin at different doses (80mg vs. 10mg) and concludes that treatment with atorvastatin 80m/day reduces the incidence of myocardial infarction and both fatal and non-fatal stroke in comparison with the administration of 10mg/day which reduces LDL-c to a lesser extent. Moreover, there is evidence on intensive therapy with rosuvastatin achieving further reduction of LDL-c in comparison with conventional treatment and the regression of aortic atheromatosis16.
In case of not achieving target LDL-c with statins at maximum doses or in case these are contraindicated, there are other lipid-lowering drugs that can be combined with the first8. Table 3 depicts additional average changes of the lipid profile achieved by different drugs when combined with statins17-21.
Bile acid sequestrants (resins) are usually badly tolerated and are not indicated in the case of mixed dyslipidemia since they can increase plasma triglycerides.
Ezetimibe (a selective cholesterol absorption inhibitor) has proven its efficacy and excellent clinical tolerability in several trials where it was used in combination with different statins22-24. It achieves an average reduction of 18% added to that of statins therefore enabling the achievement of treatment targets in a larger number of patients. A meta-analysis of 18 randomized clinical trials including 14497 patients has not concluded significant differences regarding the appearance of rhabdomyolisis or hepatotoxicity between treatment with ezetimibe and statins or statins in monotherapy25.
Another meta-analysis26 concludes that combining statins with a lower LDL-c reducing capacity with bile acid sequestrants or ezetimibe can be an alternative to monotherapy with more aggressive lipid lowering statins among high risk patients intolerant to higher doses or those who do not achieve LDL-c goal levels. The IMPROVE-IT study (inclusion criteria: stable patients following recent ACS with LDL-c levels ≤ 125 mg/dL or ≤ 100 mg/dL if previously treated with statins) has analyzed in over 18000 patients the safety profile and cardiovascular benefits of the combination statin/ezetimibe in comparison with simvastatin alone and has proved a significant reduction of LDL-c in the group receiving ezetimibe (69mg/dl vs. 53 mg/dl) and a moderate yet significant reduction of the main end point, a composite of death from cardiovascular disease, nonfatal myocardial infarction, unstable angina, coronary revascularization and nonfatal stroke, without undesirable effects (gallstones, hepatic disorders, rhabdomyolisis, myalgia and cancer)27.
IMPROVE-IT highlights that regarding secondary prevention, an increased lowering of LDL-c (from 69 to 53 mg/dl) entails a prognostic benefit attributable to the achieved reduction of LDL-c in the same line as the regression line that relates cardiovascular events to the achieved level of LDL-c in statin intervention studies. Moreover it does not detect a prognostic J-curve effect for LDL-c concentrations under 30 mg/dl.
The results of this study have practical implications since they endorse the usefulness of the combined therapy of statins and ezetimibe to, according to European guidelines8, reduce LDL-c levels and thus reduce cardiovascular mortality of very high cardiovascular risk patients. A modified therapeutic strategy has been even suggested from aggressive lipid lowering statins to aggressive cholesterol lowering treatment28 (Table 4). When reductions of LDL-c between 50 and 60% are required to meet the objectives this can be managed with more aggressive statins alone (atorvastatin and rosuvastatin) at maximum doses or with statins with a lower LDL-c reducing capacity added to ezetimibe. When reductions over 60% are required a very aggressive lipid lowering treatment is necessary with atorvastatin or rosuvastatin at maximum doses and ezetimibe 10mg.
2. Patients with Type 2 diabetes
Most of type 2 diabetes patients are among very high cardiovascular risk patients and thus LDL-c goal levels for both type 1 and type 2 diabetes patients are depicted in Table 18,9.
The main features of dyslipidemia in type 2 diabetes patients are: the presence of an excessive amount of small dense LDL particles with a not excessively increased concentration of LDL-c, increased plasma triglycerides and reduced HDL-c. The combination of these components is known as the atherogenic lipid triad. Treatment must be aimed at lowering LDL-c as the main therapeutic objective8 without ignoring strategies aimed at reducing triglycerides and increasing HDL-c when altered. In order to control atherogenic dyslipidemia optimal control of diabetes must be optimized together with appropriate control of blood pressure, the promotion of healthy dietary habits like the Mediterranean diet, regular physical exercise and stop smoking7,8.
A comprehensive approach and intensive intervention on all risk factors should be implemented in everyday clinical practice to reduce the high cardiovascular morbidity and mortality that diabetic patients present, as revealed by the STENO study29. An observational follow-up study including a cohort of diabetic patients has concluded that the control of LDL-c is the most important variable in cardiovascular prevention in these patients and has observed a higher hospitalization due to cardiovascular disease rate in patients with uncontrolled risk factors (18.2/1000 persons-year, 95% CI: 16.5-20.2) or only with HbA1C in the control (16.9, 15.0 to 19.9)30.
The 4S, CARE, LIPID and HPS studies, although not specifically designed to evaluate a diabetic population, has shown by means of subgroup analysis the reduction of cardiovascular events in type 2 diabetes patients under treatment with pravastatin or simvastatin31-35. Furthermore, another study with atorvastatin 10mg/day exclusively carried out in diabetic patients, proved a significant reduction of cardiovascular morbid-mortality36. A meta-analysis of 14 randomized clinical trials with statins in 18686 diabetic patients reported that a 1mmol/l (39gm/dl) reduction of LDL-c further reduced cardiovascular events by 21%, coronary events by 22%, and cerebrovascular events by 21% and cardiovascular mortality by 13%. Benefits were independent of initial LDL-c levels.
The use of statins in patients with diabetes or metabolic syndrome has been controversial after the results of the JUPITER study, which revealed an increased rate of new cases of diabetes in the group that received rosuvastatin 20mg/day. Nevertheless, although in patients at higher risk of developing diabetes the treatment with rosuvastatin was associated with a 28% increase of diabetes (p=0.01), a total of 134 vascular events or deaths were prevented per every 54 new cases of diabetes diagnosed38. A metaanalysis39 revealed that statin therapies were associated with a 9% increased risk of developing diabetes de novo, even higher with higher doses of statins. However, benefits in terms of reduction of cardiovascular risk, was 16% with aggressive lipid lowering strategies with statins. Therefore, it seems that cardiovascular benefits associated with statin treatment clearly outweigh the risk of developing diabetes, even in individuals at high risk of doing so.
On the other hand, the Japanese J-PREDICT study carried out in patients with impaired glucose tolerance40 proves a beneficial effect of pitavastatin on the appearance of new onset diabetes. The group which received pitavastatin 1-2 mg/day and lifestyle modifications experienced 18% reduction of the incidence of diabetes (primary end point) in comparison with the control group which only received lifestyle modification advice. This proves that the diabetogenic effect of statins does not seem a class effect and pitavastatin may have a different behavior.
With regard to the use of ezetimibe, subgroup analysis of the IMPROVE-IT study highlights the relevant benefit that this has in diabetic patients27. Another meta-analysis also proves that the lipidlowering efficacy of ezetimibe is higher among diabetic patients, with significantly higher reductions of LDL-c, total cholesterol and non HDL-c in comparison with non diabetic patient41. When goal LDLc levels are not achieved in diabetic patients with maximally tolerated doses of statins, the addition of ezetimibe may be considered8. The addition of ezetimibe further contributes to improve the percentage of patients who achieve an appropriate control of LDL-c42.
If lifestyle modifications and statin therapy do not achieve goal LDL-c levels and the disorders included in the lipid triad are still present, the addition of fibrates may be considered due to their effect on triglycerides (reduction of plasma concentration by 36%) and HDL cholesterol (increase by 10%) (Table3). Nevertheless, the FIELD43 and ACCORD44 studies did not prove a reduction of cardiovascular mortality for fenofibrate although it has proven a benefit in the atherogenic dyslipidemia patient subgroup in a meta-analysis45. European guidelines8 conclude that "fibrates and especially fenofibrate due to its low myopathic potential are indicated in combined therapy with statins to improve lipid control in patients with combined atherogenic dyslipidemia, especially in patients with metabolic syndrome".
Nicotinic acid is the drug which further increases HDL-c but the HPS2-THRIVE46 study shows that the benefit-risk balance of the combination niacin/ laropiprant is unfavorable and authorization for the commercialization of this drug has been suspended.
3. Patients with chronic kidney disease (CKD)
Statin therapy has proven to reduce cardiovascular risk in patients with chronic kidney disease (CKD)47. A meta-analysis of 11 clinical trials including 21295 participants concluded that statin therapy reduced overall mortality (p<0.0001) and both cardiovascular and cerebrovascular events (p=0.0001 and o=0.0022 respectively) in CKD not requiring dialysis. However, the use of statins in CKD patients requiring dialysis seems less robust since a non significant effect was concluded on overall mortality and on cerebrovascular events, although it did reduce deaths from cardiac disease (p<0.05) and cardiovascular episodes (p<0.05)48.
The KDIGO guidelines49 suggest that in adults with CKD requiring dialysis, treatment with statins or the combination statin and ezetimibe should not be initiated, although it is not recommended to interrupt it in patients who were already receiving this treatment when dialysis was initiated.
European guidelines on cardiovascular prevention7 consider that patients with chronic kidney disease (CKD) and a glomerular filtration rate (GFR) <30ml/ min/1.73m2 as very high risk patients and establish the same goal LDL-c levels (LDL-c under 70 mg/dl or at least a 50% reduction of the basal level) as for patients with a history of atherosclerotic vascular disease (secondary prevention). Moreover patients with CKD and GFR between 30 and 60 ml/min/1.73m2 are considered high risk cardiovascular risk patients and goal LDL-c levels are established under 100mg/ dl. In order to achieve an appropriate lipid control, statin therapy is necessary in most of these cases. The SHARP study on CKD patients proves that the combination of simvastatin and ezetimibe reduces cardiovascular morbid-mortality50.
The choice of statin therapy in CKD patients should be based on the lipid lowering efficacy and safety profile since patients with CKD present an increased incidence of muscle adverse effects. Thus potential drug interactions should be monitored in patients who are frequently polymedicated and statins eliminated mainly by the hepatic route should be preferred (atorvastatin, fluvastatin and pitavastatin) as recommended by the European guidelines for the management of dyslipidemia in CKD patients8.
4. High cardiovascular risk primary prevention patients
Cardiovascular prevention should consider the overall risk of each individual and act on each cardiovascular risk factor. The estimation of overall cardiovascular risk allows taking tailored decisions according to the risk of each patient. It identifies high risk patients that would demand earlier and more aggressive interventions than lower risk patients.
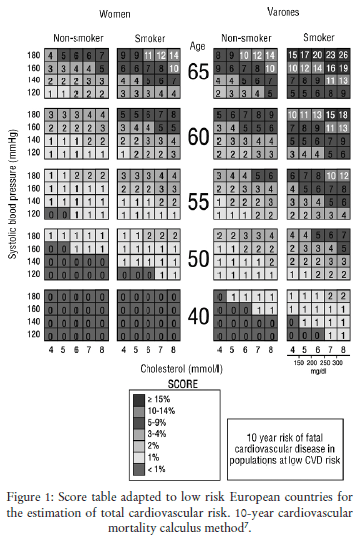
The European SCORE project (Systematic Coronary Risk Evaluation) has allowed developing an estimation system of total cardiovascular risk based on European clinical practice and considering geographical differences of that risk. It is a method of calculating 10 year cardiovascular mortality which jointly takes into account a series of risk factors (see Figure 1). The system is based on data from European cohort studies with 205,178 participants who represent a follow-up of 2.7 million people per year. Two different models were estimated, known as high and low risk, corresponding to countries with high and low incidence rates of cardiovascular diseases (CVD). European guidelines7 recommend that Spain uses SCORE tables adapted to low risk European countries (Figure 1).
The use of lipid lowering drugs in primary prevention, particularly statins, should consider if the benefit for cardiovascular prevention is enough to recommend its use. A meta-analysis51 shows that for individuals with a risk under 10% every 1 mmol/l lowering of LDL-c (39mg/dl) entails a 5-year absolute reduction of 11 per every 1000 major vascular events. However, in primary prevention the benefit of statin therapy is less robust than in other groups (secondary prevention, genetic dyslipidemias, diabetes). The efficacy of statins is risk-dependant: there is a higher benefit when the patient's cardiovascular risk is higher52.
High cardiovascular risk patients are those with SCORE estimations ranged between 5 and 10% and therapeutic goal LDL-c levels are set at under 100mg/ dl8, which will mostly require the use of statins with enough lipid-lowering power as to achieve goal control levels. If necessary, ezetimibe should be associated9.
Patients with genetic dyslipidemias such as familial hypercholesterolemia (FH) (autosomal dominant disease due to mutations of the LDL receptor gene) are considered high cardiovascular risk patients and since this disease is characterized by very high plasma concentrations of cholesterol and its deposit in several tissues, aggressive statin therapy is recommended (rosuvastatin 20 mg/day, atorvastatin 80 mg/day) which can be associated to ezetimibe and/or resins to achieve goal LDL-c levels or get as close as possible9. Early diagnosis of FH and its treatment with statins has contributed to reduce the high cardiovascular morbid-mortality of this population53. Diagnostic criteria of heterozygous familial hypercholesterolemia (MedPed/WHO) are depicted in Table 5. Definite diagnosis is established with punctuations over 8 points.
We currently count upon new drugs that can contribute to a wider therapeutic armory for the management of familial hyperlipidemias. Mipomersen inhibits the production of apo B-100 and has proven a reduction of LDL-c by 44% in patients with familial hypercholesterolemia with significantly high LDL-c in spite of maximally tolerated doses of statins. Adverse effects of this drug include flu-like symptoms, local reactions at the injection site and elevation of liver enzymes54. Lomitapide has been evaluated for homozygous familial hypercholesterolemia; at an average dose of 40mg/day it reduces LDL-c by 50% in week 26 of treatment. Gastrointestinal symptoms were the most common adverse effects55.
PCKS9 inhibitors are human monoclonal antibodies such as evolocumab, alirocumab and bococizumab that inhibit converstase subtilisin/kexin type-9 (PCSK-9) which acts as an inhibitor of cholesterol LDL receptor. They have proven reductions of LDLc of over 50% in different patient groups56. Recently, the European Medicines Agency (EMA) has recommended the approval of alirocumab and evolocumab for combined used with statins when target LDL-c levels are not achieved in patients with primary hypercholesterolemia (familial or not) or mixed dyslipidemia; or combined with other drugs when statins cannot be used57,58. They are subcutaneously administered every 2 to 4 weeks and have been trialed in patients treated with maximally tolerated doses of statins. Alirocumab added to statin therapy at maximally tolerated doses in a study which included 2341 patients (67% secondary prevention patients), reduced LDL-c by 62% and post hoc analysis evidenced a reduction of cardiovascular events59. Preliminary results from a clinical trial with evolocumab including 4465 patients (30% of which secondary prevention) show similar reduction rates of LDL-c (61%) in comparison with conventional lipid-lowering therapies as well as its capacity to reduce cardiovascular events60. The tolerance of these subcutaneous drugs has been excellent, most common effects are associated with local reactions at the injection site such as erythema, itching, edema or pain.
5. Patients with hypertriglyceridemia
Non pharmacological measures are specially relevant in the control of hypertriglyceridemia including alcohol withdrawal, low calorie intake, restriction of simple sugars and appropriate glucose control in diabetic patients. For high and very high cardiovascular risk patients, statin therapy is essential to achieve goal LDL-c levels.
However, in patients with isolated hypertriglyceridemia and appropriate control of LDL-c, the use of fibrates and/or omega 3 is indicated, particularly when plasma triglycerides are over 500mg/dl to limit the risk of pancreatitis.
n-3 fatty acids at around 3 grams/day can be an alternative for the management of hypertriglyceridemia, in the case of fibrate intolerance or contraindication. However, follow-up of a cohort of patients with multiple cardiovascular risk factors, daily treatment with n-3 fatty acids did not cardiovascular mortality and morbidity61, and a meta-analysis62 showed no statistically significant association between the administration of n-3 fatty acids and a lower risk of overall mortality, cardiac death, sudden death, myocardial infarction or stroke.
6. Polymedicated patients
It is worth considering the potential increase of adverse effects with the coadministration of statins metabolized by cytochrome P450 3A4 (atorvastatin, lovastatin and simvastatin) with other drugs eliminated by the same metabolic route9. The FDA recommends maximum doses of 20mg/day of simvastatin when administered with amlodipine, ranolazine or amiodarone and 10mg/day with verapamil or diltiazem63.
In case an association of statins and fibrates is necessary, the risk of myopathy is greater with gemfibrozil and thus its addition to statins should be avoided. Statins are a substrate for OATP1B1 transporter and gemfibrozil powerfully inhibits it. This mechanism explains the interaction between cerivastatin and gemfibrozil for which fatal rhabdomyolisis has been reported and caused the recall of this statin64.
Special mention should be made of patients infected by human immunodeficiency virus (HIV). An adverse effect of antiretroviral therapy, mainly of protease inhibitors, is the increase of plasma cholesterol and triglycerides, which strongly contributes to increase the cardiovascular risk of this population and entails systematic lipid-lowering treatment.
Highly active antiretroviral therapy (HAART) prevents the virus from multiplying and thus improves the control of the disease indefinitely. HAART inhibits several enzymes belonging to the CYP family, particularly the 3A4 isoform which increases adverse effects with the simultaneous administration of other drugs eliminated by the same route such as atorvastatin, lovastatin and simvastatin.
A FDA safety communication published in 201265, classifies pharmacological interactions between antiretroviral therapy and statins. Protease inhibitors can increase plasma concentrations of statins and thus increase the risk of myopathy. Table 6 includes FDA recommendations65, where the association between lovastatin and simvastatin and all protease inhibitors is contraindicated. Atorvastatin can be used with ritonavir at maximum doses of 20 mg/day if combined with saquinavir, fosamprenavir or darunovir. Nevertheless, the use of atorvastatin is contraindicated if ritonavir is combined with tipranavir. Anyhow, exhaustive surveillance is encouraged.
Although rosuvastatin is not eliminated by CYO 3a4, an increase of its plasma concentration has been observed when administered with ritonavir/lopinavir. The interaction could be due to the inhibition of OATP-1B1 transporter, responsible for liver capture of rosuvastatin and other statins, by protease inhibitors. The FDA recommends in this case maximum doses of 10mg/day of rosuvastatin65.
Although interaction with protease inhibitors have not been described with fluvastatin, the FDA considers that there is not enough data to recommend its prescription. Instead, the FDA indicated that the use of pitavastatin or pravastatin with protease inhibitors has no dose limitation. A pharmacokinetic study carried out on healthy volunteers has not established the lack of a relevant effect of the combination between pitavastatin and lopinavir/ritonavir on the systemic exposure of this statin66.
With regard to these patients, basal LDL-c levels, lipid-lowering capacity of each statin and its interactions with HAART will enable the choice of the most appropriate strategy to achieve therapeutic goals in each patient. Lovastatin and simvastatin would be contraindicated; atorvastatin and rosuvastatin have limitations regarding their prescription while pravastatin and pitavastatin have no dosage limitations and can be an alternative for these patients, particularly pitavastatin which has a higher lipid-lowering power and thus a greater facility to achieve lipid control goals.
HIV treatment also includes other drugs such as non-nucleoside reverse transcriptase inhibitors (NNRTI) such as delavirdine, efavirenz or neviparine which can interact with most of statins. Rosuvastatin and Pitavastatin seem the safer drugs in patients under NNRTIs. A NNRTI such as zidovudine does not present a risk of interaction with statins. Other limitation that should be considered in HIV patients is the contraindication of the administration of elvitegravir (integrase inhibitor) with lovastatin and simvastatin.
Conclusions
Dyslipidemia is one of the main cardiovascular morbidity and mortality risk factors and our therapeutic actions should be aimed at the overall cardiovascular risk rather than cholesterol alone. The introduction of statins as lipid-lowering drugs in the management of dyslipidemia has been heralded as a major breakthrough in cardiovascular prevention as many intervention studies have proven clinical benefits in the reduction of cardiovascular morbidity and mortality67,68.
Overall cardiovascular risk assessment is relevant to adapt treatment to specific LDL-c goal levels in each risk category. The final choice of a specific drug and its dosage must always take into account concomitant therapies and tolerance, patient clinical state and preferences, in an attempt to avoid clinical inertia and choose the best therapeutic option and thus improve adherence. We must not forget that lifestyle modifications remain the basis upon which cardiovascular prevention and treatment are built.
References
1. Baena-Díez JM, Félix FJ, Grau M, Cabrera de León A, Sanz H, Leal M, et al. Risk Factor Treatment and Control in Relation to Coronary Disease Risk in the Spanish Population of the DARIOS Study 2011. Rev Esp Cardiol. 2011; 64(9):766-73. [ Links ]
2. Plana N, Ibarretxe D, Cabré A, Ruiz E, Masana L. Prevalence of atherogenic dyslipidemia in Primary Care patients at moderate-very high risk of cardiovascular disease. Cardiovascular risk perception. Clin Invest Arterioscler. 2014; 26(6):274-84. [ Links ]
3. Pascual V, Ruiz E, Pintò X. Patient's care and management of dyslipidemia in type 2 diabetic patients in the clinical practice in Spain: The LIPEDIA study. Clin Invest Arterioscler. 2015; 27(2):45-56. [ Links ]
4. Banegas JR, Vegazo O, Serrano P, Luengo E, Mantilla T, Fernández R, et al. HISPALIPID Study Group Investigators. The gap between dyslipidemia control perceived by physicians and objective control patterns in Spain. Atherosclerosis. 2006; 188:420-4. [ Links ]
5. Murga N, Ruiz E, Pascual V. Patient's care and management of dyslipidemia at discharge after an acute coronary syndrome in the clinical practice in Spain: The SINCOPA study. Clin Investig Arterioscler. 2015; 27(6):272-9. [ Links ]
6. Diaz A, Murga N, Camafort-Babkowski M, Lopez JC, Ruiz E, Ruiz-Baena, et al. Therapeutic inertia in hypercholesterolaemia is associated with ischaemic events in primary care patients. A casecontrol study. Int J Clin Pract. 2014; 68(8):1001-9. [ Links ]
7. European Association for Cardiovascular Prevention & Rehabilitation, Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren WM, et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012): The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Atherosclerosis. 2012; 223(1): 1-68. [ Links ]
8. European Association for Cardiovascular Prevention & Rehabilitation, Reiner Z, Catapano AL, De Backer G, Graham I, Taskinen MR, Wiklund O, et al. ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J. 2011; 32:1769-818. [ Links ]
9. SEMERGEN (Internet). Madrid: SEMERGEN; 2015 (actualizado 2015; citado 4 Nov 2015). Documento de consenso de Sociedades Médicas de la Comunidad Valenciana para el manejo clínicopráctico de la dislipemia; (aprox. 3 p.). Disponible en: http://www.semergencv.com/docs_2/dislipemia.pdf. [ Links ]
10. Cholesterol Treatment Trialists' (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170000 participants in 26 randomised trials. Lancet. 2010; 376:1670-81. [ Links ]
11. Stein E. New statins and new doses of older statins. Curr Atheroscler Rep. 2001; 3:14-8. [ Links ]
12. Alonso Karlezi RA, Mata Pariente N, Mata López P. Control de las hiperlipemias en la práctica clínica. Rev Esp Cardiol Supl. 2006; 6 Supl G:24-35. [ Links ]
13. Alagona P Jr. Pitavastatin: Evidence for its place in treatment of hypercholesterolemia. Core Evid. 2010; 5:91-105. [ Links ]
14. Pallares V, Pascual V, Godoy-Rocati. Dislipidemia y riesgo vascular. Una revisión basada en nuevas evidencias. Semergen. 2015 Nov-Dec; 41(8):435-45. Doi: 10.1016/j.semerg.2014.10.015. [ Links ]
15. LaRosa JC, Grundy SM, Waters DD, Shear C, Barter P, Fruchart JC, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005; 352(14):1425-35. [ Links ]
16. Yogo M, Sasaki M, Ayaori M, Kihara T, Sato H, Takiguchi S, et al. Intensive lipid lowering therapy with titrated rosuvastatin yields greater atherosclerotic aortic plaque regression: Serial magnetic resonance imaging observations from RAPID study. Atherosclerosis. 2014; 232: 31-9. [ Links ]
17. Ros E. Inhibición de la absorción intestinal del colesterol: nueva diana terapéutica en la reducción de la colesterolemia. Clin Invest Arterioscl. 2003; 15 (6): 261-75. [ Links ]
18. Ansell BJ. Rationale for combination therapy with statin drugs in the treatment of dyslipidemia. Curr Atheroscler Rep. 2005; 7: 29-33. [ Links ]
19. Birjmohun RS, Hutten BA, Katelein JJP, Stroes ESG. Efficacy and safety of high-density lipoprotein cholesterol-increasing compounds. A metaanalysis of randomized controlled trials. J Am Coll Cardiol. 2005; 45:185-97. [ Links ]
20. Barter P, Ginsberg HN. Effectiveness of Combined Statin Plus Omega-3 Fatty Acid Therapy for Mixed Dyslipidemia. Am J Cardiol. 2008; 102 (8): 1040-5. [ Links ]
21. Demonty I, Ras RT, van der Knaap HC, Meijer L, Zock PL, Geleijnse JM, et al. The effect of plant sterols on serum triglyceride concentrations is dependent on baseline concentrations: a pooled analysis of 12 randomised controlled trials. Eur J Nutr. 2013; 52(1):153-60. [ Links ]
22. Ballantyne CM, Abate N, Yuan Z, King TR, Palmisano J. Dose-comparison study of the combination of ezetimibe and simvastatin (Vytorin) versus atorvastatin in patients with hypercholesterolemia: the Vytorin Versus Atorvastatin (VYVA) study. Am Heart J. 2005; 149:464-73. [ Links ]
23. Ballantyne CM, Weiss R, Moccetti T, Vogt A, Eber B, Sosef F, et al. EXPLORER Study Investigators. Efficacy and safety of rosuvastatin 40mg alone or in combination with ezetimibe in patients at high risk of cardiovascular disease (results from the EXPLORER study). Am J Cardiol. 2007; 99:673-80. [ Links ]
24. Pearson TA, Denke MA, McBride PE, Battisti WP, Brady WE, Palmisano J. A community-based, randomized trial of ezetimibe added to statin therapy to attain NCEP ATP III goals for LDL cholesterol in hypercholesterolemic patients: the ezetimibe add-on to statin for effectiveness (EASE) trial. Mayo Clin Proc. 2005; 80:587-95. [ Links ]
25. Kashani A, Sallam T, Bheemreddy S, Mann DL, Wang Y, Foody JM, et al. Review of side effect profile of combination ezetimibe and statin therapy in randomized clinical trials. Am J Cardiol. 2008; 101:1606-13. [ Links ]
26. Gudzune KA, Monroe AK, Sharma R, Ranasinghe PD, Chelladurai Y, Robinson KA. Effectiveness of Combination Therapy With Statin and Another Lipid-Modifying Agent Compared With Intensified Statin Monotherapy: A Systematic Review. Ann Intern Med. 2014; 160(7):468-76. [ Links ]
27. Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, et al. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N Engl J Med. 2015; 372(25):2387-97. [ Links ]
28. Masana L, Pedro-Botet J, Civeira F. IMPROVEIT clinical implications. Should the "high-intensity cholesterol-lowering therapy" strategy replace the "high-intensity statin therapy"? Atherosclerosis 2015; 240(1):161-2. [ Links ]
29. Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003; 348(5):383-93. [ Links ]
30. Nichols GA, Joshua-Gotlib S, Parasuraman S. Independent contribution of A1C, systolic blood pressure, and LDL cholesterol control to risk of cardiovascular disease hospitalizations in type 2 diabetes: An observational cohort study. J Gen Intern Med. 2013; 28:691-7. [ Links ]
31. Haffner SM, Alexander CM, Cook TJ, Boccuzzi SJ, Musliner TA, Pedersen TR, et al. Reduced coronary events in simvastatin-treated patients with coronary heart disease and diabetes or impaired fasting glucose levels: subgroup analyses in the Scandinavian Simvastatin Survival Study. Arch Intern Med. 1999; 159: 2661-7. [ Links ]
32. Goldberg RB, Mellies MJ, Sacks FM, Moye LA, Howard BV, Howard WJ, et al. The Care Investigators: Cardiovascular events and their reduction with pravastatin in diabetic and glucose-intolerant myocardial infarction survivors with average cholesterol levels: subgroup analyses in the cholesterol and recurrent events (CARE) trial. Circulation. 1998; 98: 2513-9. [ Links ]
33. Keech A, Colquhoun D, Best J, Kirby A, Simes RJ, Hunt D, et al. LIPID Study Group. Secondary prevention of cardiovascular events with long-term pravastatin in patients with diabetes or impaired fasting glucose: results from the LIPID trial. Diabetes Care. 2003; 26(10):2713-21. [ Links ]
34. Nichols GA, Joshua-Gotlib S, Parasuraman S. Independent contribution of A1C, systolic blood pressure, and LDL cholesterol control to risk of cardiovascular disease hospitalizations in type 2 diabetes: an observational cohort study. J Gen Intern Med. 2013; 28(5):691-7. [ Links ]
35. Collins R, Armitage J, Parish S, Sleigh P, Peto R. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003; 361: 2005-16. [ Links ]
36. Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004; 364: 685-96. [ Links ]
37. Cholesterol Treatment Trialists' (CTT) Collaborators. Efficacy of cholesterol-lowering therapy in 18 686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet. 2008; 371:117-25. [ Links ]
38. Preiss D, Seshasai SR, Welsh P, Murphy SA, Ho JE, Waters DD, et al. Risk of incident diabetes with intensive-dose compared with moderatedose statin therapy: a meta-analysis. JAMA. 2011; 305(24):2556-64. [ Links ]
39. Ridker PM, Pradhan A, MacFadyen JG, Libby P, Glynn RJ. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: an analysis from the JUPITER trial. Lancet. 2012 A; 380(9841):565-71. [ Links ]
40. Odawara M, Yamazaki T, Kishimoto J, Ito C, Noda M, Terauchi Y, et al. Effect of pitavastatin on the incidence of diabetes in Japanese individuals with impared glucose tolerance. 49th European Association for the Study of the Diabetes Annual Meeting; 2013 Sept. 23-27; Barcelona, Spain. Available from: http://www.easdvirtualmeeting.org/resources/3645. [ Links ]
41. Leiter LA, Betteridge DJ, Farnier M, Guyton JR, Lin J, Shah A, et al. Lipid-altering efficacy and safety profile of combination therapy with ezetimibe/ statin vs. statin monotherapy in patients with and without diabetes: an analysis of pooled data from 27 clinical trials. Diabetes, Obesity Metabolism 2011; 13:615-28. [ Links ]
42. Maron DJ, Hartigan PM, Neff DR, Weintraub WS, Boden WE; COURAGE Trial Investigators. Impact of Adding Ezetimibe to Statin to Achieve Low-Density Lipoprotein Cholesterol Goal (from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) Trial). Am J Cardiol. 2013; 111:1557-62. [ Links ]
43. Keech A, Simes RJ, Barter P, Best J, Scott R, Taskinen MR, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005; 366(9500):1849-61. [ Links ]
44. ACCORD Study Group, Ginsberg HN, Elam MB, Lovato LC, Crouse JR 3rd, Leiter LA, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010; 362(17):1563-74. [ Links ]
45. Sacks FM, Carey VJ, Fruchart JC. Combination lipid therapy in type 2 diabetes. N Engl J Med. 2010; 363:692-4. [ Links ]
46. HPS2-THRIVE Collaborative Group. HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur Heart J. 2013; 34(17): 1279-91. [ Links ]
47. Hou W, Lv J, Perkovic V, Yang L, Zhao N, Jardine MJ, et al. Effect of statin therapy on cardiovascular and renal outcomes in patients with chronic kidney disease: a systematic review and meta-analysis. Eur Heart J. 2013; 34:1807-17. [ Links ]
48. Barylski M, Nikfar S, Mikhailidis DP, Toth PP, Salari P, Ray KK, et al. Lipid and Blood Pressure Meta-Analysis Collaboration Group. Statins decrease all-cause mortality only in CKD patients not requiring dialysis therapy--a meta-analysis of 11 randomized controlled trials involving 21,295 participants. Pharmacol Res. 2013; 72:35-44. [ Links ]
49. Kidney Disease: Improving Global Outcomes (KDIGO) Lipid Work Group. KDIGO Clinical Practice Guideline for Lipid Management in Chronic Kidney Disease. Kidney inter. 2013; 3 Suppl: 259-305. [ Links ]
50. Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, et al. SHARP Investigators. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): A randomised placebo-controlled trial. Lancet. 2011; 377:2181-92. [ Links ]
51. Cholesterol Treatment Trialists' (CTT) Collaborators. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012; 380(9841):581-90. [ Links ]
52. Minder CM, Blumenthal RS, Blaha MJ. Statins for primary prevention of cardiovascular disease: the benefits outweigh the risks. Curr Opin Cardiol. 2013; 28(5):554-60. [ Links ]
53. Raal FJ, Pilcher GJ, Panz VR, van Deventer HE, Brice BC, Blom DJ, et al. Reduction in Mortality in Subjects With Homozygous Familial Hypercholesterolemia Associated With Advances in Lipid-Lowering Therapy. Circulation. 2011; 124: 2202-7. [ Links ]
54. Frishman W. Ricotta DN. Mipomersen: a safe and effective antisense therapy adjunct to statins in patients with hypercholesterolemia. Cardiol Rev. 2012; 20(2):90-5. [ Links ]
55. Cuchel M1, Meagher EA, du Toit Theron H, Blom DJ, Marais AD, Hegele RA, et al. Efficacy and safety of a microsomal triglyceride transfer protein inhibitor in patients with homozygous familial hypercholesterolaemia: a single-arm, openlabel, phase 3 study. Lancet. 2013; 381(9860):40-6. [ Links ]
56. Zhang XL, Zhu QQ, Zhu L, Chen JZ, Chen QH, Li GN, et al. Safety and efficacy of anti-PCSK9 antibodies: a meta-analysis of 25 randomized, controlled trials. BMC Med. 2015; 13:123. [ Links ]
57. European Medicines Agency. Praluent recommended for approval to lower cholesterol. Medicine to offer therapy for patients unable to control high cholesterol with currently available treatment (Internet). London: EMA; 2015. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_ events/news/2015/07/news_detail_002377.jsp&mid=WC0b01ac058004d5c1. [ Links ]
58. European Medicines Agency. First-in-class treatment to lower cholesterol. Repatha to offer therapy for patients unable to control high cholesterol with currently available treatment (Internet). London: EMA; 2015. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2015/05/news_detail_002336.jsp&mid=WC0b01ac058004d5c1. [ Links ]
59. Robinson JG, Farnier M, Krempf M, Bergeron J, Luc G, Averna M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015; 372(16):1489-99. [ Links ]
60. Sabatine MS, Giugliano RP, Wiviott SD, Raal FJ, Blom DJ, Robinson J, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015; 372:1500-9. [ Links ]
61. The Risk and Prevention Study Collaborative Group. N Engl J Med. 2013; 368:1800-8. [ Links ]
62. Rizos EC1, Ntzani EE, Bika E, Kostapanos MS, Elisaf MS. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis. JAMA. 2012; 308(10):1024-33. [ Links ]
63. US Food and Drug Administration (Internet). Silver Spring: FDA; 2015 (updated 2011 Dec 15; cited 2015 Nov 4). FDA Drug Safety Communication: New restrictions, contraindications, and dose limitations for Zocor (simvastatin) to reduce the risk of muscle injury; (about 2 screens). Available from: http://www.fda.gov/Drugs/DrugSafety/ucm256581.htm. [ Links ]
64. Shitara Y, Hirano M, Sato H, Sugiyama Y. Gemfibrozil and its glucuronide inhibit the organic anion transporting polypeptide 2 (OATP2/ OATP1B1:SLC21A6)-mediated hepatic uptake and CYP2C8-mediated metabolism of cerivastatin: analysis of the mechanism of the clinically relevant drug-drug interaction between cerivastatin and gemfibrozil. J Pharmacol Exp Ther. 2004; 311:228-36. [ Links ]
65. US Food and Drug Administration (Internet). Silver Spring: FDA; 2015 (updated 2012 Feb 28; cited 2015 Nov 4). FDA Drug Safety Communication: Important safety label changes to cholesterol-lowering statin drugs. (about 4 screens). Available from: http://www.fda.gov/Drugs/DrugSafety/ucm293101.htm. [ Links ]
66. Morgan RE, Campbell SE, Suehira K, Sponseller CA, Yu CY, Medlock MM. Effects of steady-state lopinavir-ritonavir on the pharmacokinetics of pitavastatin in healthy adult volunteers. JAIDS 2012; 60(1):158-64. [ Links ]
67. Cholesterol Treatment Trialists' (CTT) Collaboration, Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010; 376: 1670-81. [ Links ]
68. Cholesterol Treatment Trialists' (CTT) Collaboration, Fulcher J, O'Connell R, Voysey M, Emberson J, Blackwell L, Mihaylova B, et al. Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet. 2015; 385: 1397-405. [ Links ]
![]() Correspondence:
Correspondence:
Dr. Vicente Pascual Fuster.
Email: pascual_vic@gva.es
Text received: 28/11/2015
Text accepted: 30/11/2015