Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Enfermería Global
versão On-line ISSN 1695-6141
Enferm. glob. vol.21 no.66 Murcia Abr. 2022 Epub 02-Maio-2022
https://dx.doi.org/10.6018/eglobal.485851
Originals
Evaluation of a peripheral vein for intravenous chemotherapy: a prospective observational study
1 MSc, RN. Departamento de Oncología, Hospital Universitario de Brasilia, Brasilia, DF, Brasil
2 PhD, Profesor investigador. Departamento de Medicina de Rehabilitación, Facultad de Medicina, Universidad de Washington, Seattle, WA. Estados Unidos de América.
3 Rn, Hospital Base del Distrito Federal, Brasilia, DF, Brasil
4 PhD, RN, Profesor Adjunto Facultad de Enfermería, Universdad Federal de Minas Gerais, Belo Horizonte, MG, Brasil
5 PhD, RN, Profesor Asociado, Departamento de Enfermería, Facultad de Ciencias de la Salud, Universidad de Brasilia, Brasilia, DF, Brasil
This study aimed to assess over time alterations of a peripheral vein used for chemotherapy infusion in patients with breast cancer. It is a prospective observational study which included patients who were scheduled to receive peripheral infusion of chemotherapy. These patients had the first peripheral vein used for infusion evaluated in five moments: before the venipuncture, after device removal at the end of the first chemotherapy infusion, and on days 21, 42, and 63 after the first infusion. The primary outcome was the caliber of the vein, measured in millimeters with a Veinlite LEDX® transilluminator and a tape measure. Fifty-nine women receiving doxorubicin and docetaxel for the first time were enrolled to the study. The caliber size varied from 2 to 4 millimeters at baseline, and decreased overtime. During the follow-up period, peripheral veins of 35 women (59.3%) were measured at 0 mm at day 63. The remaining 24 women (40.7%) had some recovery, but for 15 of them (62.5%) the vein became a palpable cord. The feasibility of using a peripheral vein to perform chemotherapy decreased as the treatment progresses.
Keywords: antineoplastic agents; breast neoplasms; infusions, intravenous; nursing care
INTRODUCTION
Most chemotherapy protocols for breast cancer are available only in intravenous administration, and generally they contain irritant and/or vesicant drugs. Intravenous chemotherapy administered peripherally causes damage to the vascular endothelium of the peripheral venous network, sometimes causing complications such as phlebitis, infection, and tissue necrosis due to chemotherapy extravasation1. These complications might lead to anxiety, needle phobia, refusal of treatment, and emotional distress of the patient2,3.
In women with breast cancer, difficulty in obtaining venous access for chemotherapy infusion is a frequent problem. These women usually undergo at least four cycles of neoadjuvant chemotherapy, followed by surgery and further cycles of adjuvant chemotherapy, and access to venous network becomes a problem as the treatment progress. This problem is exacerbated when the patient has unilateral mastectomy with axillary lymphadenectomy because, in this case, only the contralateral arm can be used for venipuncture and chemotherapy infusion4. When the patient has bilateral mastectomy, the problem is even worse, because both arms might have their lymphatic drainage compromised, making it difficult to access and maintain the patency of the venous network.
Breast cancer patients frequently need to receive antibiotics, hydration, or even blood transfusion during the intervals between cycles of chemotherapy protocols. When a peripheral vein cannot be used, a new vein is punctured and over time, as the treatment progresses, there will be fewer options for available peripheral veins, which may cause stress to the nursing staff attending the patient. If no peripheral veins are viable, the patient might have to delay the chemotherapy treatment, or be hospitalized to have a central venous access installed, or have an emergency procedure to access a deep vein.
Therefore, we need to understand the trajectory of the conditions of peripheral veins in women with breast cancer receiving chemotherapy through peripheral administration. To our knowledge, there are no studies in the literature on the natural history of the damage caused by chemotherapy to the venous network. The aim of this study was to evaluate the alterations over time of a peripheral vein used one single time for chemotherapy infusion in breast cancer patients.
METHODS
Design
This was a prospective observational study conducted at the Center of High Complexity in Oncology in a teaching hospital in Brazil. This study adheres to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement.
Population and Sample
Women who had been diagnosed with breast cancer and were scheduled to receive doxorubicin and docetaxel by peripheral intravenous route were eligible for this study. Further inclusion criteria included: 18 years or older and undergoing chemotherapy for the first time in their lives. Exclusion criteria were: presence of peripheral vascular disease, previous axillary lymphadenectomy or radiotherapy to axilla, previous intravenous antibiotic therapy, and current use of anticoagulant therapy. In addition, the patient’s first venipuncture had to be in the forearm, dorsal surface of the hand, or wrist. If the only accessible vein was the antecubital vein, the patient was not eligible for this study.
During six months, all women who were scheduled to receive the chemotherapy protocol were invited to participate in the study by one of the investigators (S.L.N.S.). Inclusion and exclusion criteria were checked, and the objectives and procedures of the study were explained to the patient.
Since the primary objective of this study was to evaluate the magnitude of problems with a peripheral vein used for infusion, no hypothesis tests were involved, and no previous studies on the topic have been found in the literature, a formal sample size calculation was not warranted. We aimed to include as many participants as possible during the time frame of the study, in order to have robust estimation of the magnitude of problems with vein accessibility.
Ethical Considerations
The study was approved by the Committee of Ethics in Research of the School of Health Sciences of the University of Brasilia (approval n.063/2011). The participants were given a detailed explanation of the study procedures and written informed consent was obtained from each participant.
Procedures
Participants underwent four cycles of neoadjuvant chemotherapy using doxorubicin and docetaxel drugs, and were evaluated at each chemotherapy cycle. The cycle consisted of one day of chemotherapy (CHT) infusion, followed by 20 days of rest. On the day of the first cycle, a nurse would choose a vein for venipuncture according to accessibility in the following order: forearm (distal to proximal direction), dorsal surface of the hand, and wrist. The vein was evaluated before the venipuncture (denominated “Before CHT”) and after the removal of the peripheral catheter at the end of the chemotherapy infusion (“After CHT”). The vein that was punctured on that day was not punctured again for CHT, but it was evaluated on the next CHT cycles at days 21, 42 and 63 after the first venous infusion (which we denoted by D21, D42, D63, respectively).
Outcomes
We collected data on vein caliber, palpability, visibility and rectilinearity. These outcomes were chosen because they are clinically important when a venipuncture is necessary. Also, at each evaluation, the woman was asked if she was hospitalized between chemotherapy cycles and if that specific vein was used during hospitalization.
A Veinlite LEDX® transilluminator was used to visualize the vein and the caliber was measured in millimeters by using a tape measure. The transilluminator is a C-shaped ring of 31 mm of diameter, based on cold light source (24 oranges and 8 red ultra-bright LED lights), which maximizes imaging contrast during transillumination.
Statistical Analysis
Descriptive analysis was performed for all variables. For vein caliber over time, the number and proportion of participants in each trajectory was calculated. Confidence intervals with 95% confidence were calculated for the proportions of certain outcomes (such as, proportion of women who had 0 mm of vein caliber at day 63, for example). Data were analyzed using IBM SPSS (version 26.0).
RESULTS
Fifty-nine women receiving doxorubicin and docetaxel for the first time were enrolled in the study. Age ranged from 23 to 65 years old (mean = 46.0, SD = 10.1).
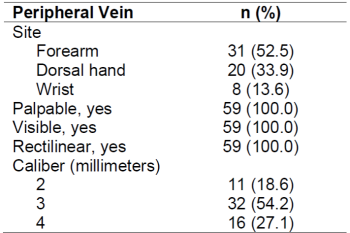
Table 1 shows the distribution of the sites and characteristics of the peripheral vein that was accessed by venipuncture for the first chemotherapy infusion. Superficial veins of the forearm were chosen for venipuncture in 52.5% of the patients, followed by superficial veins of dorsal hand (33.9%) and wrist (13.6%). All veins were palpable, visible, and rectilinear. Measurement of veins was in millimeters, rounded up to the closest millimeter, and it varied from 2 to 4 millimeters, with the majority having 3 mm (54.2%).
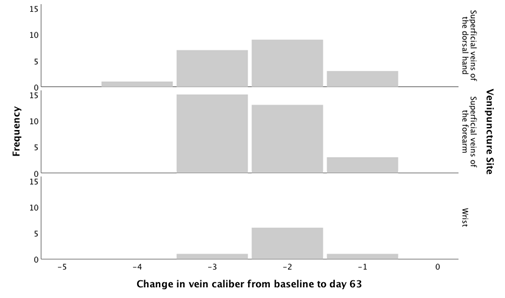
Table 2 shows the trajectory of the peripheral vein caliber during the follow-up period. None of the veins returned to their baseline caliber during the follow-up time. In 24 women (40.7%, 95% confidence interval [CI]: 28% - 53%), the vein caliber had some recuperation, but in 35 women (59.3%, 95% CI: 47% - 72%) the vein caliber was 0 mmeven at the last follow-up on day 63. From the 24 women whose veins had some recovery, 15 of them (62.5%, 95% CI: 43% - 82%) had sclerosed veins with a palpable cord that was nonfunctional for venous access. The damage to the vein occurred regardless of the vein caliber size at the baseline or the venipuncture site (Figure 1).
Table 2. Caliber size of peripheral vein during follow-up time (n=59)
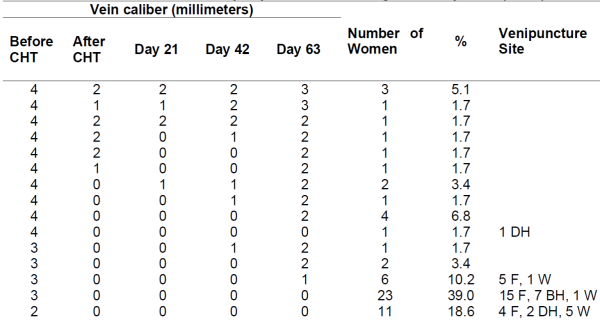
CHT = Chemotherapy, F = Forearm, DH = Dorsal Hand, W = Wrist
Sum of percentages is not 100% due to rounding error.
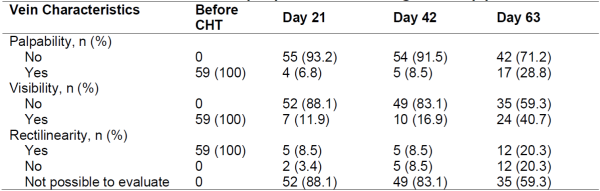
The characteristics of the veins regarding palpability, visibility and rectilinearity are described in Table 3. Most of the veins became unpalpable and invisible after the first cycle of chemotherapy. The majority of the veins did not recover their palpability (71.2%, 95% CI: 60%- 83%) or visibility (59.3%, 95% CI: 47% - 72%) on D63.
During the follow-up period, 12 women were hospitalized for clinical management, specifically to receive intravenous antibiotic therapy (n = 6) or hydration (n = 6) due to neutropenia and emesis related to the chemotherapy protocol. Most of them were hospitalized between the baseline and D42. The studied veins were not used during the hospitalization period, thus not affecting our results.
DISCUSSION
This study aimed to evaluate the alterations over time of a peripheral vein used one single time for neoadjuvant chemotherapy infusion in breast cancer patients. Fifty-nine women received doxorubicin and docetaxel for the first time. We observed a large proportion of women whose vein reduced in caliber over the study period, including many whose vein remained at 0 mm to the end of the study.
Despite the increase of availability of oral chemotherapy agents, the intravenous route is still the most used for infusion of antineoplastic chemotherapy. Neoadjuvant chemotherapy is common in breast cancer treatment and it is used to reduce the size of the tumor before surgery, increasing the likelihood of better outcomes5. However, the intrinsic characteristics of the chemotherapy agents cause damage to the vascular endothelium. Chemotherapy protocols are usually performed in many cycles, thus leading to an overload of the peripheral venous network.
Doxorubicin and docetaxel are drugs well-known for their cardio and vascular toxicity6. These drugs usually lead to endothelial dysfunction induced by oxidative stress and induction of proinflammatory cytokines that leads to the increase of vasoconstriction7,8. During the follow-up period, most of peripheral veins had caliber of 0 mm even at the last follow-up on day 63. A large number of women experienced palpable cord in their veins, which might be explained by the endothelial apoptosis known to be caused by taxanes9.
Multiple unsuccessful attempts at venipuncture may limit future peripheral vein access3)(10)(11)(12). In addition, there is a risk of increased peripheral chemotherapy extravasation in veins that had had multiple cannulation attempts, with incidence reported in the literature from 0.08% to 6.5%13,14. Although the incidence is low, the devastating long-term effects of chemotherapy extravasation on the patient can result in significant pain, tissue necrosis, and loss of function at the site of injury13. Therefore, superficial veins of the forearm are preferable for the venipuncture, with smaller risk of injury given that they are far from articulations. Neoadjuvant chemotherapy protocols start with vesicant drugs, and the forearm site is usually chosen for venipuncture in the beginning of the treatment. In this study, women who started the chemotherapy protocol in the forearm veins had them damaged, and that would have reduced the options for venipuncture site in the next cycles of chemotherapy.
Alternative routes of infusion should be explored to conserve the peripheral veins in breast cancer patients, since they will have a long treatment that requires intravenous access. According to the literature, if the patient repeatedly requires more than three venipuncture attempts, and the treatment plan is to undergo chemotherapy for an extend period of time, a central venous catheter may be appropriate15,16.
Central venous access is mandatory for a number of specific solutions for infusion, such as those containing vesicant drugs. Chemotherapy for breast cancer usually has at least one vesicant drug in the protocol. There are complications related to central venous catheter and also to peripheral veins catheter, but the choice of a peripheral vein device in Brazilian public hospitals is usually justified based on the low cost. Among the patients evaluated in this study, 20 (27%) had a central venous catheter inserted in some moment of the adjuvant therapy. Therefore, if a patient will have a central venous catheter implanted at some point of his/her treatment, on the long run, it might be less costly and less damaging to the patient to have the central catheter inserted at the beginning of the chemotherapy. There is a need to analyze the long-term cost-benefit of using a central venous catheter versus peripheral venous devices when performing chemotherapy in those patients.
Limitations
In this study, a tape measure was used as an instrument to measure the caliber of peripheral veins. The authors suggest that the micrometer be used as the device for measuring the caliber of veins in future studies. Since the micrometer is an instrument that can measure millimeters more accurately, its use can identify caliber recovery smaller than 1 millimeter which may be clinically important.
CONCLUSION
As the treatment progresses, the vascular endothelium toxicity caused by chemotherapy agents decreases the accessibility of peripheral veins for chemotherapy in women with breast cancer. The magnitude of the problem is large and can be damaging to the patient and her treatment. Alternative routes, such as a central venous catheter, should be explored to conserve the veins for future use. This is important especially in breast cancer patients, whose treatment is very long and requires intravenous access.
REFERENCIAS
1. Bertoglio S, Boxtel TV, Goossens GA, Dougherty L, Furtwangle R, Lennan E, et al. Mejora de los resultados del acceso vascular periférico corto en la administración de oncología y quimioterapia. J Vasc Access. 2017;18(2), 89-96. Disponible en: https://doi.org/10.5301%2Fjva.5000668 [ Links ]
2. Reis PED, Rodrigues CC, Vasques CI, Carvalho EC. Efectos adversos por Drogas quimioterápicas identificados en el sitio de infusión intravenosa periférica. Ciencia y Enfermeria. 2008;14(2), 55-64. [ Links ]
3. McGowan D, Wood S. Desarrollando una herramienta de evaluación venosa en la administración de quimioterapia intravenosa. Br J Nurs. 2008;17(3), 158-164. Disponible en: https://doi.org/10.12968/bjon.2008.17.3.28404 [ Links ]
4. Cole T. Riesgos y beneficios del uso de agujas en pacientes después de una cirugía de ganglios axilares. Br J Nurs. 2006;15(18), 969-979. Disponible en: https://doi.org/10.12968/bjon.2006.15.18.22020 [ Links ]
5. García-Mata J, García-Palomo A, Calvo L, Mel R, Cruz JJ, Ramos M. Estudio de fase II de doxorrubicina densa en dosis y docetaxel como quimioterapia neoadyuvante con apoyo de G-CSF en pacientes con cáncer de mama grande o localmente avanzado. Clin Transl Oncol. 2008;10(11), 739-744. Disponible en: https://doi.org/10.1007/s12094-008-0280-z [ Links ]
6. Montañez-Valverde RA, Hurtado-de-Mendoza D, Loaiza-Bonilla A. Cardiotoxicidad: Miocardio o Endotelio. Cureus. 2018;10(7), e2994. Disponible en: https://doi.org/10.7759/cureus.2994 [ Links ]
7. Cameron AC, Touyz RM, Lang NN. Complicaciones vasculares de la quimioterapia contra el cáncer. Can J Cardiol. 2016;32(7), 852-862. Disponible en: https://doi.org/10.1016/j.cjca.2015.12.023 [ Links ]
8. Soultati A, Mountzios G, Avgerinou C, Papaxoinis G, Pectasides D, Dimopoulos MA, et al. Toxicidad vascular endotelial por agentes quimioterapéuticos: evidencia preclínica e implicaciones clínicas. Cancer Treat Rev. 2012;38(5), 473-483. Disponible en: https://doi.org/10.1016/j.ctrv.2011.09.002 [ Links ]
9. Wojcik T, Szczesny E, Chlopicki S. Efectos perjudiciales de la quimioterapia y otros fármacos en el endotelio: un llamado a la elaboración de perfiles de toxicidad endotelial. Pharmacol Rep. 2015;67(4), 811-817. [ Links ]
10. Mestre G, Berbel C, Tortajada P, Alarcia M, Coca R, Fernández MM, et al. Intervención multifacética exitosa dirigida a reducir los eventos adversos relacionados con el catéter venoso periférico corto: un estudio de cohorte cuasi experimental. Am J Infect Control. 2013; 41(6), 520-526. Disponible en: https://doi.org/10.1016/j.ajic.2012.07.014 [ Links ]
11. Alexandrou E, Ray-Barruel G, Carr PJ, Frost S, Inwood S, Higgins N, et al. Prevalencia internacional del uso de catéteres intravenosos periféricos. J Hosp Med. 2015; 10(8), 530-533. Disponible en: https://doi.org/10.1002/jhm.2389 [ Links ]
12. Atay S, Sen S, Cukurlu D. Cateterismo venoso periférico relacionado con la flebitis y los factores de riesgo asociados. Niger J Clin Pract. 2018; 21(7), 827-831. Disponible en: https://doi.org/10.4103/njcp.njcp_337_17 [ Links ]
13. Schulmeister L. Manejo de extravasación: actualización clínica. Semin Oncol Nurs. 2011; 27(1), 82-90. Disponible en: https://doi.org/10.1016/j.soncn.2010.11.010 [ Links ]
14. Sakaida E, Sekine I, Iwasawa S, Kurimoto R, Uehara T, Ooka Y, et al. Incidencia, factores de riesgo y resultados del tratamiento de la extravasación de agentes citotóxicos en una clínica de quimioterapia para pacientes ambulatorios. Jpn J Clin Oncol. 2014; 44(2), 168-171. Disponible en: https://doi.org/10.1093/jjco/hyt186 [ Links ]
15. Gallieni M, Pittiruti M, Biffi R. Acceso vascular en pacientes oncológicos. CA: Cancer J Clin. 2008; 58(6), 323-346. Disponible en: https://doi.org/10.3322/ca.2008.0015 [ Links ]
16. Gorski L, Hadaway L, Hagle ME, McGoldrick M, Orr M, Doellman D. Estándares de práctica de la terapia de infusión. J Infus Nurs, 2016; 39(1S), 1-169. Disponible en: https://doi.org/10.1097/nhh.0000000000000481 [ Links ]
Received: July 07, 2021; Accepted: November 28, 2021











 texto em
texto em