My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Enfermería Global
On-line version ISSN 1695-6141
Enferm. glob. vol.22 n.71 Murcia Jul. 2023 Epub Nov 13, 2023
https://dx.doi.org/10.6018/eglobal.554941
Originals
Validation of the "International Consultation on Incontinence Questionnaire - Urinary Incontinence Short Form (ICIQ-UISF)" for the Portuguese population
1Catholic University of Portugal (UCP), Institute of Health Sciences, Center for Interdisciplinary Research in Health (CIIS). Porto, Portugal
2Hospital Center of Vila Nova de Gaia/Espinho (CHVNG), Gynecology Service. Vila Nova de Gaia, Portugal
Introduction:
Urinary incontinence can be considered a common condition associated with the aging process with extremely disabling symptoms, being two to three times more prevalent in women than in men. The use of validated instruments for the initial diagnosis of urinary incontinence is essential to obtain uniformity and systematization in the assessment of this condition. Objective: To adapt and validate the International Consultation Questionnaire Urinary Incontinence Short Form (ICIQ-UI SF) for the Portuguese population.
Methodology:
Two independent translations of the ICIQ-UI SF were performed by Portuguese translators fluent in English. After harmonizing them, the resulting translation was independently back translated by two English translators fluent in Portuguese. The final version of the ICIQ-UI SF into Portuguese was applied to 90 users of the outpatient urology, urology and gynecology inpatient clinic of a hospital located in Gaia. The psychometric properties and reliability of the questionnaire were evaluated.
Results:
No changes to the original format of the ICIQ-UI SF were observed at the end of the instrument's translation and adaptation process. The average age of participants was 55 years. Internal consistency was high, as demonstrated by Cronbach's alpha coefficient (0.85). Pearson's coefficient for questions 3 and 4 was 0.88 and for questions 4 and 5 it was 0.82. The evaluation was considered satisfactory and statistically significant.
Conclusion:
The Portuguese version of the ICIQ-UI SF was successfully validated, making it possible to apply it to the Portuguese population with satisfactory reliability and construct validity.
Keywords: urinary incontinence; surveys and questionnaires; validation study; translating
INTRODUCTION
Urinary incontinence is a common condition with a significant negative impact on people's quality of life(1)). The "International Continence Society" (ICS) defines UI as a complaint of involuntary loss of urine(2). The implications of this definition for health in general are extensive, as it implies that any involuntary loss of urine is within this definition. Urinary incontinence can be considered a common condition associated with the aging process with extremely disabling symptoms, being two to three times more prevalent in women than in men(3),(4). Despite not being a life-threatening condition, it may have a major negative impact on quality of life, developing progressively and burdensomely. Urinary incontinence implies significant indirect costs, associated with social isolation, high emotional burden, direct costs related to expenses on medical devices for urine containment and to carry out treatments(5).
The use of validated instruments for the initial diagnosis of urinary incontinence is essential to achieve standardization and systematization in the assessment of this condition. The "International Consultation on Incontinence Questionnaire Urinary Incontinence Short Form" (ICIQ-UI-SF) is recommended for use, both at scope of clinical practice and in research(6). It is a simple and short questionnaire with six questions that allow assessing the presence of loss of urine, its frequency, severity and identifying the typology of these same loss of urine, allowing to assess the overall impact on quality of life, having been developed by International Continence Society. The original version is in English but it has been translated into different languages including Brazilian Portuguese(7), but not into the Portuguese language and culture. It can be used for the initial diagnosis, during the treatment stage and for patient follow-up, being a self-administered questionnaire, but can also be completed by a health professional(8),(9). It presents a score to be calculated from the sum of questions three, four and five, from zero to 21, in which the higher the score, the worse the condition perceived by the patient.
The objective of the study is the translation, cultural adaptation and validation of the ICIQ-UI SF into the Portuguese language and culture for its use in research and clinical practice in Portugal.
METHODOLOGY
The methodological study was conducted in two stages. The first stage was the translation and back-translation of the instrument and the assessment of content validity. The second stage comprised the evaluation of psychometric properties. The internal consistency of the Portuguese version of the ICIQ-UI SF was evaluated by using Cronbach's alpha. Construct validity was obtained through exploratory factor analysis. To identify and characterize the presence of urinary incontinence, the ICIQ-UI SF is the questionnaire indicated by the ICS as the most appropriate for its assessment(9). It is a simple, short, and self-administered questionnaire, quickly assessing the type of Urinary Incontinence and the overall impact on quality of life. It has 6 (six) simple questions that assess the frequency, severity and overall impact on quality of life of urinary incontinence for research and clinical practice purposes. The estimated total response time is 2 to 3 minutes, the questions are simple and without cultural content, with scores from 0-21 where zero means that there is no loss of urine and 21 refers to the most severe situation. Including two questions about the amount and frequency of urine loss, the third question that assess the impact of urine loss in general on quality of life, and the last question is to characterize the type of urine loss, and more than one option can be chosen, and the first two questions allow to identify the participant's age and sex.
In the first stage of the study, translation and back-translation were performed according to the protocol by Guillemin et al(10) which has five steps: initial translation, synthesis of the translation, back-translation, committee of experts and pre-test of the final version. The procedure started after authorization from the ICIQ study group by Nikki Cotterill, with two independent translations performed by two Portuguese translators fluent in English. One, with previous knowledge on the aim of the study and from the field of health, and the second without knowledge from the field of health and the aim of the study. After analysis and harmonization of the two versions, the resulting version was submitted to back-translation by two English translators fluent in Portuguese, without knowledge of the objectives of the study and of the original questionnaire. After analysis and harmonization of the back-translation with the original in English, the Portuguese translation was considered grammatically and semantically equivalent to the original version and able to be submitted to a group of three Portuguese experts in the field of health (nurses with clinical and academic experience) with mastery of the English language. After this evaluation, the Portuguese version of the ICIQ-UI-SF was pre-tested, in a convenience sample of ten patients from the outpatient urology consultation at Centro Hospitalar de Vila Nova de Gaia/Espinho, inviting participants to complete the instrument and comment on their understanding. The time that each participant took to answer the questionnaire was monitored, verifying that it took about two minutes to be completed. No difficulties were identified in completing the questionnaire.
In the second stage of the study, a cross-sectional observational study was conducted on a convenience sample consisted of users, of both sexes, who sought the Urology consultation service, the Urology inpatient service and the Gynecology service of the Centro Hospitalar Vila Nova de Gaia/Espinho E.P.E., from September to December 2019. Users over 18 years old and who could read Portuguese, with or without urinary incontinence, were invited to participate.
The study was approved by the Ethics Committee of the Centro Hospitalar Vila Nova de Gaia/Espinho E.P.E. under registration 99/2019-2.
In the approach to the participants, the study was explained and their written consent to participate was requested in accordance with the Helsinki principles(11). The completion of the ICIQ-UI SF was requested at the end of the nursing consultation or during hospitalization either in the Urology service or in the Gynecology service. The ICIQ-UI SF is a self-administered questionnaire that assesses the overall impact of UI on quality of life, allowing the qualification of urinary loss through four questions, assessing the frequency and severity of loss expressed by patients.
The sample size was determined in view of the initial instrument validation study, thus considering a sample of ninety participants(9) given that Bryman and Cramer(12) state that the number of sample elements should be at least five times the number of items on the scale, so it was considered an appropriate sample for validation.
A descriptive analysis was made using the frequencies of categorical variables and measures of position and dispersion of continuous variables.
As a measure of reliability, the internal consistency of the instrument, and the intraclass correlation coefficient were evaluated. For internal consistency, it was used the standardized Cronbach's alpha coefficient; the scale's sensitivity was measured using Spearman's correlation, a test used to analyze the relationships between variables. The significance level adopted was 5% for a 95% Confidence Interval. The software used was the Statistical Package for the Social Sciences (SPSS) version 26 for Windows.
RESULTS
In order to evaluate the metric characteristics of the Portuguese version of the ICIQ-UI SF, the construct validity and reliability were analyzed.
Interviews were conducted with ninety participants, with different complaints at the urological level, and the sample consisted of 81.15% (n=73) female participants and 18.9% (n=17) male participants. The average age of the sample was 55 years old (± 15.8 SD), with a minimum age of 24 years and a maximum age of 94 years old. In this initial sample of 90 participants, the prevalence of urinary incontinence was 64.4% (n=58).
In the study population, according to the definition of urinary incontinence by the International Continence Society, which considers it as a complaint of any involuntary loss of urine(13), it was found in the sample those who reported at least one episode of loss of urine (n= 58), 19% (n=11) were male and 81% (n=47) female, with an average age of 58.9 years (± 14.2 SD), with a minimum age of 24 years and a maximum age of 94 years, as in the total sample of 90 participants.
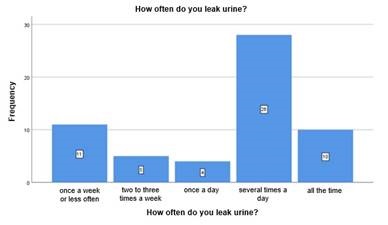
By answering to the question "How often do you leak urine?" we present the results considering the population with loss of urine (Chart 1).
In the population sample that reported having urine loss (n=58), 17.2% (n=17.21) indicated losing urine all the time, 48.3% (n=28) reported losing urine several times a day, 6.9% (n=4) reported about once a day, 8.6%(n=5) two to three times a week, and 19%(n=11) about once a week or less often.
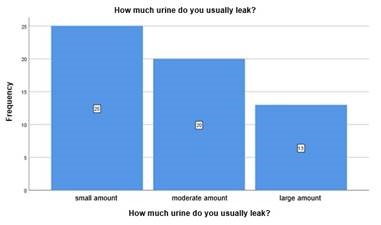
In the question "How much urine do you usually leak?", from the population sample that reported having urine loss, 43.1%(n=25) indicated a small amount, 34.5%(n=20) indicated a moderate amount and 22.4% (n=13) reported a large amount (Chart 2)
In question 5, where the question addresses whether urine loss affects daily life, it was found that urine loss is referred to as having an impact on quality of life, on a scale ranging from zero (not at all) to 10 (a lot). In the sample of those who have urine loss, 51.7% (n=30) refer to an impact on quality of life between 8 and 10 of which 16 refer to having urine loss several times a day and 8 refer to having urine loss all the time, noting that 4 have urine loss once a week or less. Regarding the amount of urine loss, 28% (n=7) of those who report a loss of a small amount indicate that it has an impact on quality of life between 8 and 10, and 20% (n=5) considered that this small loss has an impact on quality of life between 5 and 7. From the participants who reported a moderate amount of urine loss, 55% (n=11) considered that it had an impact on quality of life between 8 and 10. Overall 51.7% (n=30) indicated that urine loss had an impact on quality of life between 8 and 10.
In the question about the circumstances of urine loss, in which there may be more than one answer, 39.7% (n=23) of the sample reported urine loss before getting to the toilet, 67.2% (n= 39) reported urine loss when coughing or sneezing, 37.9% (n=22) reported urine loss when exercising, 29.3% (n=17) reported urine loss when sleeping, 27.6%(n =16) reported urine loss for no obvious reason and 13 (22.4%) had urine loss all the time.
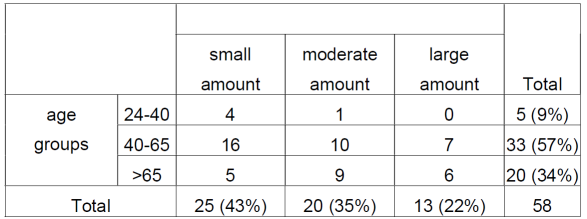
Regarding the amount of urine lost with the age groups, considering the sample of those who lose urine (n=58), it is observed that in the age group from 24 to 40 years old, being 9% of the sample, 80% reported urine loss in small amount, in the age group from 40 to 65 years old, 57% of the sample, 49% reported losing urine in a small amount, 30% reported losing urine in a moderate amount and 21% reported losing a large amount. In the age group over 65 years old, constituting 34% of the sample, 25% of the participants reported losing urine in a small amount, 45% reported losing urine in a moderate amount and 30% in a large amount (Table 1).
Participants who reported losing several times a day or all the time and when they lost moderate to large amounts of urine generally reported a worse negative impact on quality of life.
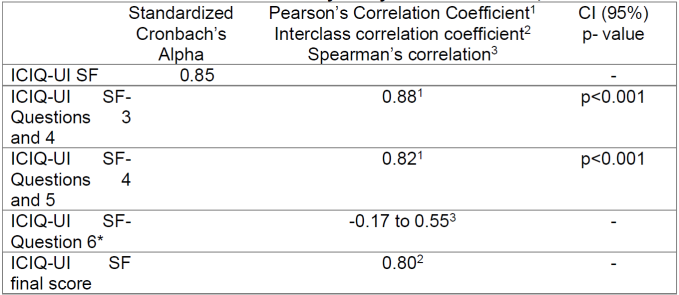
The reliability study is showed in Table 2.
As a measure of reliability, the standardized Cronbach's Alpha was 0.85 and the interclass correlation coefficient was 0.80 for a p<0.001. The Kaiser-Meyer-Olkin measured value of sample adequacy was 0.82 and Bartlett's test of sphericity was statistically significant (P<0.001). The interclass correlation coefficient calculated for the final score of the scale was 0.80 and Spearman's with a result between -0.17 to 0.55. For questions 3 and 4 and questions 4 and 5, Pearson's correlation coefficient was calculated, which had a result of 0.88 and 0.82 respectively for a p<0.001.
DISCUSSION
This study aimed to translate and culturally adapt the ICIQ-UI SF to Portuguese. The decision for the translation and cultural adaptation of this instrument was because it allows assessing the urinary incontinence and characterizing it regarding its frequency, amount, classification in terms of type and assessment of the overall impact on quality of life(9).
In 2004, the International Continence Society presented a review of 36 population studies conducted in 17 countries where the estimated prevalence for urinary incontinence at the overall level was 5 to 69%, with a prevalence of 64.4%(14).
Dolan, in their study on the impact of urinary incontinence on quality of life during pregnancy and after delivery, identified that this impact was influenced by the type of urinary incontinence and the amount of coexisting urinary symptoms(15). In this study, it was found that the severity of urinary incontinence symptoms is not always associated with a greater impact on quality of life.
The reliability, measured by internal consistency and evaluated by standardized Cronbach's alpha, was calculated by encompassing questions 3, 4 and 5(16). This coefficient allows verifying the homogeneity of the instrument. As a general rule, its value should not be less than 0.80, if the instrument is widely used, however, values above 0.60 indicate the presence of consistency(17). Cronbach's alpha was considered satisfactory with a value of 0.85. This reveals that there is a good degree of correlation between the three used questions. Pearson's coefficient for questions 3 and 4 was 0.88 and for questions 4 and 5 it was 0.82, which indicates that there is a strong positive correlation between them, the interclass correlation coefficient, for the final ICIQ score was 0.80. The ICIQ-UI SF is part of an international project called "ICIQ Modular Questionnaire". As limitations of the study, it is verified that regarding the population sample, despite having n=90 participants, only 58 participants reported urine loss, which, for the analysis of the validity of the questions about urine loss, however, when analyzing the general results obtained, there is agreement with the results obtained in the validation of the ICIQ-SF for Portuguese(16).
The Portuguese full version of the ICIQ-UI-SF is in the Figure below.
CONCLUSION
The ICIQ-UI SF version was successfully translated and validated for Portuguese, according to the result of the measurement properties analysis. This instrument, with a short and simple structure, allows the measurement of symptoms associated with the loss of urine and its overall impact on quality of life. One of the limitations of this study is related to the sample size and specially to gender heterogeneity. It is considered necessary further studies that can contribute to increase the knowledge of this phenomenon in the Portuguese population. This instrument, given its simplicity and brevity, will allow its use in clinical and research context in Portugal, enhancing the identification of people with urine loss in order to get the appropriate treatment.
REFERENCES
1. Hunskaar S, Burgio K, Diokno A, Herzog AR, Hjälmås K, Lapitan MC. Epidemiology and natural history of urinary incontinence in women. Urology. 2003;62(4 SUPPL. 1):16-23. [ Links ]
2. Wein AJ. Re.: An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. J Urol. 2011;185(5):1812. [ Links ]
3. Irwin DE, Kopp ZS, Agatep B, Milsom I, Abrams P. Incontinence and Bladder Outlet Obstruction. 2010;2-8. [ Links ]
4. Azuma R, Murakami K, Iwamoto M, Tanaka M, Saita N, Abe Y. Prevalence and risk factors of urinary incontinence and its influence on the quality of life of Japanese women. Nurs Heal Sci. 2008;10(2):151-8. [ Links ]
5. O'Dell KK, Labin LC. Common Problems of Urination in Nonpregnant Women: Causes, Current Management, and Prevention Strategies. J Midwifery Women's Heal. 2006;51(3):159-73. [ Links ]
6. Raz S, Rodríguez L. Female Urology. Female Urol. 2008;(January). [ Links ]
7. Tadeu Nunes Tamanini J, Dambros M, Arturo Levi CD, César Rodrigues Palma P, Rodrigues Netto Jr N, Tadeu Nunes Tamanini Rua Floriano Peixoto J. Validation of the "International Consultation on Incontinence Questionnaire-Short Form" (ICIQ-SF) for Portuguese [Internet]. Vol. 38, Rev Saúde Pública. 2004. Available from: https://www.fsp.usp.br/rsp [ Links ]
8. Klovning A, Avery K, Sandvik H, Hunskaar S. Comparison of two questionnaires for assessing the severity of urinary incontinence: The ICIQ-UI SF versus the incontinence severity index. Neurourol Urodyn [Internet]. 2009 Jun 1 [cited 2020 Jun 12];28(5):411-5. Available from: http://doi.wiley.com/10.1002/nau.20674 [ Links ]
9. Avery K, Donovan J, Peters TJ, Shaw C, Gotoh M, Abrams P. ICIQ: A brief and robust measure for evaluating the symptoms and impact of urinary incontinence. Neurourol Urodyn. 2004;23(4):322-30. [ Links ]
10. Guillemin F, Bombardier C, Beaton D. Cross-cultural adaptation of health-related quality of life measures: Literature review and proposed guidelines. J Clin Epidemiol. 1993;46(12):1417-32. [ Links ]
11. Kong H, West S. Declaração de Helsínquia da Associação Médica Mundial Princípios Éticos para a Investigação Médica em Seres Humanos. 2008;1-4. [ Links ]
12. Bryman, Adam; Duncan C. Quantitative Data Analysis with IBM SPSS 17 18 & 19: A Guide for Social Scientist. Routledge: Taylor & Francis Group; 2011. [ Links ]
13. Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. The standardisation of terminology in lower urinary tract function: Report from the standardisation sub-committee of the International Continence Society. Urology. 2003;61(1):37-49. [ Links ]
14. Buckley BS, Lapitan MCM. Prevalence of urinary incontinence in men, women, and children-current evidence: Findings of the fourth international consultation on incontinence. Urology [Internet]. 2010;76(2):265-70. Available from: http://dx.doi.org/10.1016/j.urology.2009.11.078 [ Links ]
15. Dolan LM, Walsh D, Hamilton S, Marshall K, Thompson K, Ashe RG. A study of quality of life in primigravidae with urinary incontinence. Int Urogynecol J. 2004;15(3):160-4. [ Links ]
16. Nunes Tamanini JT, Dambros M, D'Ancona CAL, Rodrigues Palma PC, Rodrigues Netto N. Validation of the "International Consultation on Incontinence Questionnaire - Short Form" (ICIQ-SF) for Portuguese. Rev Saude Publica. 2004;38(3):438-44. [ Links ]
17. Souza AC de, Alexandre NMC, Guirardello E de B. Propriedades psicométricas na avaliação de instrumentos: avaliação da confiabilidade e da validade. Epidemiol e Serv saude Rev do Sist Unico Saude do Bras. 2017;26(3):649-59. [ Links ]
Acknowledgements
The authors would like to thank nurses Marta Inês Pereira Passos, Júlia Seixas and Rosa Albuquerque for their collaboration in data collection.
Received: January 23, 2023; Accepted: March 05, 2023











 text in
text in