Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Medicina Oral, Patología Oral y Cirugía Bucal (Internet)
versión On-line ISSN 1698-6946
Med. oral patol. oral cir.bucal (Internet) vol.11 no.5 ago./sep. 2006
ORAL MEDICINE AND PATHOLOGY
The relationship between the levels of salivary cortisol and the presence of xerostomia in menopausal women. A preliminary study
Relación entre los niveles de cortisol salival y la presencia de xerostomía en mujeres menopaúsicas. Estudio preliminar
Begoña Rivera Gómez 1, Gonzalo Hernández Vallejo2, Lorenzo Arriba de la Fuente 3, Margarita López Cantor4, Milagros Díaz 3, Rosa Mª López-Pintor 4
(1) Especialista Universitario en Medicina Oral. Becaria Predoctoral de Investigación
(2) Profesor Titular
(3) Profesor Asociado
(4) Especialista Universitario en Medicina Oral. Profesor del Título de Especialista Universitario en Medicina Oral.
Departamento de Medicina y Cirugía Bucofacial.
Facultad de Odontología. UCM
ABSTRACT
Xerostomia is a particularly frequent occurrence among menopausal women, and is often associated with depression.
Objectives: To evaluate the relationship between unstimulated salivary flow rate and the presence of xerostomia, and to determine the levels of salivary cortisol and its relationship with xerostomia.
Study design: Thirty women were selected from patients attending the Department of Medicine and Buccofacial surgery, and formed into two groups, study and control. Samples of unstimulated salivary flow were collected, and the amounts of salivary cortisol determined using the ELISA technique (enzyme-linked immunosorbent assay).
Results: The mean unstimulated salivary flow rates for the control and study group were 0.37 ± 0.28 ml/min and 0.24 ± 0.18 ml/min, respectively. The concentration of salivary cortisol was 3.47 ± 1.64 ng/ml for the control group and 2.29 ± 2.60 ng/ml for the study group. The statistical tests applied showed no significant differences for either variable between the two groups in the study.
Conclusions: The results of the present study indicate that there is no relationship between variations in the rates of unstimulated salivary flow and the corresponding concentration of cortisol.
Key words: Xerostomia, hyposalivation, unstimulated salivary flow, cortisol, depression, menopause.
RESUMEN
La xerostomía es un síntoma especialmente frecuente en las
mujeres en torno a la menopausia, que se asocia en muchas ocasiones con estados depresivos.
Objetivos: Valorar la relación entre las cifras del flujo salival total no estimulado y la presencia de xerostomía, y determinar los niveles de cortisol salival y su relación con la misma.
Diseño del estudio: Se seleccionaron 30 mujeres de los pacientes que acuden al Departamento de Medicina y Cirugía Bucofacial y se formaron dos grupos, estudio y control. Se recogieron muestras de flujo salival total no estimulado y mediante técnica de ELISA se determinaron las cifras de cortisol salival.
Resultados: Las medias de flujo salival total no estimulado para el grupo control y estudio fueron de 0.37± 0.28 ml/min. y de 0.24± 0.18 ml/min. respectivamente. La concentración de cortisol salival fue de 3.47± 1.64 ng/ml. para el grupo control y de 2.29± 2.60 ng/ml. para el grupo estudio. Las pruebas estadísticas aplicadas no mostraron diferencias significativas entre los dos grupos del estudio para ambas variables.
Conclusiones: Los resultados del presente estudio indican que no existe relación entre las variaciones en el flujo salival total no estimulado y las concentraciones de cortisol correspondientes.
Palabras clave: Xerostomía, hiposialia, flujo salival total no estimulado, cortisol, depresión, menopausia.
Introduction
Dry mouth is a relatively common symptom. When we examine the literature, we see a wide variation in the data, which offers figures for prevalence of between 14 and 46%, being consistently higher among women (1-3). Dry mouth is found to be more common within the older population, where its frequency oscillates between 13 and 39% for those able to look after themselves, and increasing up to 60% in institutionalized or hospitalized individuals (3,4). Changes in the salivary glands are related to age; however there is no evidence that xerostomia is solely a result of the aging process (5). Of the different causes observed (6), those more frequently associated with xerostomia are menopause (7,8) and certain depressive conditions (9,10). Menopause is defined as the permanent (after 12 months) cessation of menstruation resulting from the loss of ovarian function. The age at which physiological menopause appears is between 45 and 55 years, with an average of 52.5 years (11). Many menopausal women suffer changes in mood (12), especially depressive disorders, which have been associated with xerostomia (9, 10, 13), although the causal relationship between these two factors and dry mouth is unclear.
With regard to depression and its relationship with the appearance of xerostomia, various studies have used different tests to evaluate the psychological condition, establishing a direct relationship between these variables, both with the appearance of the symptomatology and with its disappearance on the improvement of the depression (9,10).
Studies that evaluate the levels of cortisol in patients suffering from depression have shown variable results, with notable differences between these studies. While some authors indicate that a deficit in cortisol is related with chronic episodes of depression, others note that elevated levels of endogenous glucocorticoids may cause psychiatric disorders such as depression (14-16). These latter authors also indicate that older patients, a condition particularly related to xerostomia (17), and children are more vulnerable to the effects of high levels of glucocorticoids on brain function. In general terms, differences in salivary cortisol seem to exist between depressed patients and healthy individuals (15,18). For this reason, cortisol levels could be used as a biological marker for changes related to anxiety and depression, and although its predictive value is controversial, this possible relationship would be a relevant finding for the multidisciplinary management of these patients.
Based on the above, and due to the high prevalence of xerostomia in menopausal women with mood disorders, the purpose of this study was to evaluate if those patients with a subjective sensation of dry mouth presented modifications in salivary cortisol. In addition, and given the relationship
between this hormone and depressive states, to compare these patients with those who did not refer such a sensation. Therefore, the objectives we stated were: to evaluate the relationship between the rates of unstimulated salivary flow and the presence of xerostomia in menopausal women, and to determine the levels of salivary cortisol and its relationship with the sensation of a dry mouth.
Material and method
Thirty patients, with informed consent, were selected by non-probable sample from those attending our department. Two groups were formed, study and control, with 15 patients in each group. Selection criteria for the study group were: female, aged 45 or older, cessation of menstruation for at least 12 months, hormonal analysis compatible with menopause and a diagnosis of xerostomia (Table 1). The control group was constituted by patients meeting the same criteria with the exception of a diagnosis of xerostomia.
Exclusion criteria included all clinical symptoms of xerostomia produced by specific causes. Likewise, patients under treatment for xerostomia were also excluded; as were those taking natural or synthetic corticosteroids for prolonged periods due to the suppression of the hypothalamus-hypophysis-adrenal system produced (Table 2).
The salivary flow was measured for all patients included in the study. To obtain the samples of unstimulated salivary flow, specifically designed collection tubes graduated in millimeters were used, and labeled according to a system of letters and numbers, the letter indicated the group to which the patient belonged and the number indicating the order in which the samples were taken. Prior to taking the sample, the patient had a mouthwash of water in order to eliminate any possible detritus and so obtain a clean sample. The patient was then instructed to deposit at intervals all the saliva accumulated over a period of 5 minutes into the tube. Once the sample had been obtained it was allowed to settle, placing the tube in a test tube rack, in order to achieve a better reading of the saliva volume.
After taking the reading, the samples were sent to the laboratory where they were stored until the cortisol was determined. This was carried out using the ELISA technique (enzyme linked immunoabsorbent assay), the principal of which is based on the competition between the cortisol (antigen) and an enzyme-labeled antigen to bind with an antibody situated on the microplates. To carry out this assay, the specifically developed DiaMetra® kit for the detection of cortisol in saliva was used.
The statistical analysis was carried out on the data obtained using the SPSS 11.0 statistical package. For the comparison of both mean variables for each group the students t test was used. Due to the impossibility of eliminating from the study all those patients taking potentially xerostomizing medication, since this would create an insufficient sample size, a Pearsons correlation coefficient and a multivariate analysis were made in order to analyze the above mentioned confusion factor. To this end the two groups were subdivided into 5 subgroups according to the particular medication being taken by each patient, numbered from 1 to 5 as follows: group 1 anxiolytics, sedatives and hypnotics; group 2 antihypertensives; group 3 analgesics; group 4 antidepressants; group 5 others (bronchodilators, antihistamines).
Results
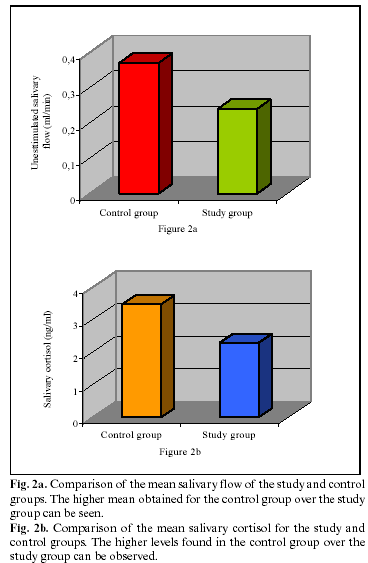
The concentration of unstimulated salivary flow (mean and standard deviation) of all the samples was 0.30±0.24 ml/min. The salivary flow rates for the study and control groups (mean and standard deviation) were 0.24±0.18 ml/min and 0.37±0.28 ml/min respectively. However, although the control group presented higher figures than the study group, these results were not statistically significant (p=0.18). This difference can be seen in Figure 1a, which shows the distribution of the position values for both groups.
The total concentration of salivary cortisol (mean and standard deviation) was 2.89±2.17 ng/ml. The total concentration of salivary cortisol for the study and the control groups was 2.29±2.60 ng/ml and 3.47±1.64 ng/ml respectively. This difference, as for the salivary flow, was not statistically significant (p=0.16).
Figure 1b shows the distribution of the position values for the total concentration of salivary cortisol for the two groups in the study where this difference can be observed.
The study group presented lower salivary flow rates than the control group, although the control group presented higher levels of concentration of cortisol (fig 2a and
2b). With regard to the mean salivary flow corresponding to the different pharmacological groups, neither the study nor the control groups showed statistically significant differences between them (p>0.05) (fig.3). The multivariate analysis for the different groups offered values of p>0.05, that is to say, there were no statistically significant differences in the modifications of unstimulated salivary flow between the study and control groups in relation to the medications being taken.
Discussion
According to the International Dental Federation (IDF), 50% of the over 40-50-year-old population present an objective decrease in salivary flow, or hyposalivation, a figure which increases up to 70% when referring to the population over 70 years of age (19).
The mean unstimulated salivary flow rates for the study and control group in our study were 0.24 ml/min and 0.37 ml/min respectively, figures which can be considered as normal for a population of this age (1); however, there were no statistically significant differences between the groups. Therefore, and in agreement with other authors, we can say that the decrease in the rate of salivary flow is not an essential condition for the development of the sensation of a dry mouth (17). In this respect, Thomson et al. in a study carried out on a population of 939 people, found that the prevalence of xerostomia and hyposalivation were 20.5 and 22.1% respectively; and that only 5.7% of the total population in that study presented both characteristics. The distribution by sex showed that 8.1% of the women had both conditions, while only 3.1% of the men referred xerostomia and also presented a decrease in salivary flow (4). Likewise, Pajukoski et al. affirmed that the subjective sensation of a dry mouth, xerostomia, is not necessarily associated with hyposalivation (20). This association between xerostomia and hyposalivation is not a constant. Xerostomia can occur in spite of the existence of correct glandular function and normal salivary flow rates, as appears in our study and confirmed in the studies of Ship et al., who observed that healthy postmenopausal women, the principal risk group (6), did not present a deterioration in glandular function nor a reduction in salivary flow. On the other hand, Närhi et al. (17), as other authors (21), affirmed that a decrease in salivary flow rate does exist following menopause (8, 17), however, they did not find statistically significant differences in the unstimulated salivary flow rates between subjects with dry mouth and the controls.
Pharmaceutical treatment plays an important role in the reduction of the unstimulated salivary flow (1,22). With respect to the most common medications, it would seem that the antihypertensives are those that generate the highest hyposalivation (22), although drugs such as tranquilizers, sedatives, antipsychotics, hypnotics and some antidepressants are also associated with a reduction in stimulated salivary flow (1). In our study, the results demonstrate that there is no correlation between the different groups of drugs classically considered as causing xerostomia, and modifications in the salivary flow rates. Likewise, on analyzing the relationship between these subgroups no statistically significant differences appear among them (p>0.05). This important finding should be interpreted with caution, given the small sample size (n=30), it would therefore be interesting to carry out a similar study on a larger population in order to increase the external validity of the results.
This data would seem to suggest that the taking of medication does not have a constant effect in relation to the development of hyposalivation, which could be due to certain individual characteristics of susceptibility (23,24). This would explain, to a certain extent, why of all individuals taking a certain medication, only some refer xerostomia. The same occurs with hyposalivation, since not all the subjects taking potentially xerostomizing medication present an objective reduction in the salivary flow.
The sensation of a dry mouth is related to the presence of increased levels of anxiety and/or certain alterations in mood, such as depression (9,10). Depression is a disorder in which a series of etiopathogenic elements: genetic, somatic, psychic and sociocultural factors, among others, can affect a predisposed personality (25). A theory which combines these factors is the so-called stress-diathesis model, according to which some people inherit a susceptibility to develop depression, possibly due to the fact that their monoaminergic systems are hypoactive, their hypothalamus-hypophysis- adrenal cortex systems are hyperactive or to a combination of both (26). Cortisol, a glucocorticoid synthesized by the adrenal cortex, plays a vital role in stressful situations by blocking the production and liberation of multiple hormones and neurotransmitters, which, in the absence of such control can lead to a state of shock. Furthermore, glucocorticoids are known to have a complex effect on the brain (25).
Since the beginning of the 20th century, it has been recognized that saliva is a biological fluid that constitutes a non-invasive method for detecting a wide range of hormones, drugs and antibodies (27,28). Of all the determinations that can be performed in saliva, that of cortisol is able to exactly and efficiently quantify the biologically active cortisol, and therefore constitutes an adequate method for evaluating the response to stress in humans (29). Moreover, the amount of salivary cortisol adequately represents the plasmatic figures (14), which, when linked to the minimal invasiveness of this technique could favor its substitution of the plasmatic technique in the future.
In agreement with the above, Hill and Walker (18), found in increased amount of salivary cortisol in those patients with higher levels of anxiety, measured on the HAD (Hospital Anxiety Depression) scale. In addition, Galard et al. (14,15) also found an increase in the levels of salivary cortisol in patients with depression.
The mean concentrations of salivary cortisol for the control and the study group obtained in this study were 3.47 and 2.29 ng/ml respectively. Although the amounts of cortisol were higher in the control group than in the study group, the differences were not statistically significant.
In the literature, the relationship between the levels of salivary cortisol and the presence of certain mood disorders such as depression (32), and stress are amply documented (33); however, within the field of oral medicine references are scarce. Specifically, there are only two studies which evaluate the amounts of salivary cortisol in patients with lichen planus (34,35) and one in patients with recurrent aphthous stomatitis (36), entities which have both been related with stress. While some authors (34,36) find an association between these pathologies and variations in the levels of salivary cortisol, others (35) on the contrary affirm that no such relationship exists. With respect to the association between the variations in levels of salivary cortisol in patients with xerostomia, Johnson et al. (37), found low levels in patients with xerostomia who suffered from Sjögrens syndrome in comparison with healthy controls. In our study, perhaps the fact that we excluded patients with Sjögrens syndrome from the study group may be the reason for the discrepancy between our data and those of other authors. Furthermore, another factor to be taken into consideration is the sample size, which may be insufficient to determine significant differences between the groups.
The results of the present study do not demonstrate the existence of a statistically significant relationship between the variations in unstimulated salivary flow and the corresponding concentrations of salivary cortisol. Likewise, there is no correlation between the taking of medications and a reduction in salivary flow, and therefore other possible etiologic agents should be investigated. The frequent appearance of certain mood disorders (depression) in conjunction with xerostomia, is such that we believe it would be interesting to carry out further studies on a larger series of patients and to also evaluate this last variable (depression), through specifically designed psychological tests, with the aim of clarifying its role in the development of xerostomia.
![]() Address for correspondence:
Address for correspondence:
Dra. Begoña Rivera Gómez
Departamento de Medicina y Cirugía Bucofacial.
Facultad de Odontología. UCM.
Ciudad Universitaria s/n
28040 Madrid
E-mail: brivera@eresmas.com
Received: 8-05-2004
Accepted: 25-05-2006
References
1. Närhi TO, Meurman JH, Ainamo A, Nevalainen JM, Schmidt-Kaunisaho KG, Siukosaari P, et al. Association between salivary flow rate and the use of systemic medication among 76-, 81-, and 86 year-old inhabitants in Helsinki, Finland. J Dent Res 1992;71:1875-80. [ Links ]
2. Locker D. Xerostomia in older adults: a longitudinal study. Gerodontol 1995;12:18-25. [ Links ]
3. Österberg T, Landahl S, Hedgard B. Salivary flow, saliva pH, and buffering capacity in 70-year-old men and women. J Oral Rehabil 1984; 11:157-70. [ Links ]
4. Thomson WM, Chalmers JM, Spencer AJ, Ketabi M. The ocurrence of xerostomia and salivary gland hypofunction in a population-based sample of older South Australians. Spec Care Dentist 1999;19:20-3. [ Links ]
5. Vissink A, Spijkervent FK, Van Nieuw Amerongen A. Aging and saliva: a review of the literature. Spec Care Dentist 1996;16:95-103. [ Links ]
6. Cawson RA, Gleeson MJ, Eveson JW. Functional disorders: xerostomia and drooling. En: Cawson RA, Gleeson MJ, Eveson JW, eds. Pathology and surgery of the salivary glands. Oxford. Ed. Isis Medical Media 1997. p. 64-80. [ Links ]
7. Tarkkila L, Linna M, Tiitinen A, Lindqvist C, Meurman JH. Oral symptoms at menopause-the role of hormone replacement therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2001;92:276-80. [ Links ]
8. Zachariasen RD. Oral manifestations of Menopause. Compendium 1993;14:1586-91.
[ Links ]9. Bergdahl J, Bergdahl M. Low unstimulated salivary flow and subjective oral dryness: association with medication, anxiety, depression and stress. J Dent Res 2000;79:1652-8. [ Links ]
10. Bergdahl J, Bergdahl M. Depressive symptoms in individuals with idiopatic subjective dry mouth. J Oral Pathol Med 1997;26:448-50. [ Links ]
11. WHO. Research on the menopause. Report of a WHO Scientific Group. Geneva. 1981. Technical Report Series No. 670:90. [ Links ]
12. Takamatsu K, Kasuga M, Makita K, Nozawa S. Evaluation of depressive conditions among Japanese patients at a menopause clinic. J Obstet Gynaecol Res 2004;61:62-70. [ Links ]
13. Mathew RJ, Weinman M, Claghorn JL. Xerostomia and sialorrhea in depression. Am J Psychiatry 1979;136:1476-7. [ Links ]
14. Galard R, Gallart JM, Catalán R, Schwartz S, Arguello JM, Castellanos JM. Salivary cortisol levels and their correlations with plasma ACTH levels in depressed patientes before and after the DST. The American Journal of Psychiatry 1991;148:505-8. [ Links ]
15. Galard R, Catalán R, Castellanos JM, Gallart JM. Plasma corticotropin-releasing factor in depressive disorders. Biol Psychiatry 1998;44: 15-20. [ Links ]
16. Barrou Z, Thomopoulos P, Luton JP. Assay of salivary cortisol. An interesting method for exploring the adrenal cortex. Presse Medicale 1997;26:329-31. [ Links ]
17. Närhi TO. Prevalence of Subjective Feelings of Dry Mouth in the Elderly. J Dent Res 1994;73:20-5. [ Links ]
18. Hill CH, Walker RV. Salivary cortisol determinations and selfrating scales in the assesment of stress in patients undergoing the extraction of wisdom teeth. Br Dent J 2001;191:513-5. [ Links ]
19. López Jornet P, Castejón Estebán Y, Bravo Ruiz C, Henarejos Hernández JL. La prevalencia de la xerostomía en la población mayor de 65 años. Odontoestomatología 2000;3:3-9. [ Links ]
20. Pajukoski H, Meurman JH, Halonen P, Sulkava R. Prevalence of subjective dry mouth and burning mouth in hospitalized elderly patients and outpatients in relation to saliva, medication, and systemic diseases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2001;92:641-9. [ Links ]
21. Parvinen T, Larmas M. Age dependency of stimulated salivary flor rate, pH, and lactobacillus and yeast concentracions. J Dent Res 1982; 61:1052-5. [ Links ]
22. Pujol T, Coma M, Pujol M, Postigo P. Prevalencia de xerostomía en la población general. Aten Primaria 1998;21:225-8. [ Links ]
23. Hattis D, Banati P, Goble R. Distribution of individual susceptibility aomng humans for toxic effects. How much protection does the traditional tenfold factor provide for what fraction of wich kinds of chemical and effects?. Ann NY Acad Sci 1999;895:286-316. [ Links ]
24. Primohamed M, Park BK. Genetic susceptibility to adverse drug reactions. Trends Pharmacol Sci 2001;22:298- 305. [ Links ]
25. Farreras P, Rozman C, eds. Medicina Interna. Madrid: Mosby-Doyma Libros; 1996. p. 1597-99. [ Links ]
26. Pinel JPJ, ed. Biopsicología. Madrid: Prentice Hall; 2001. p. 560-72. [ Links ]
27. Tabak LA. A Revolution in Biomedical Assesment: The Depelopment of Salivary Diagnostics. J Dent Educ 2001;65:1335-9. [ Links ]
28. Yao JK, Moss HB, Kirillova GP. Determination of Salivary Cortisol by Nonisotopic Immunoassay. Clinical Biochemistry 1998;31:187-90. [ Links ]
29. Mirasoli M, Deo SK, Lewis JC, Roda A, Daunert S. Bioluminescence inmunoassay for cortisol using recombinant aequorin as a label. Anal Biochem 2002;306:204-11. [ Links ]
30. Lamey PJ, Lamb AB. The usefulness of the HAD scale in assessing anxiety and depression in patients with burning mouth syndrome. Oral Surg Oral Med Oral Pathol 1989;67:390-2. [ Links ]
31. Paterson AJ, Lamb AB, Clifford TJ, Lamey PJ. Burning mouth syndrome: the relationship between the HAD scale and parafunctional habits. J Oral Pathol Med 1995;24:289-92. [ Links ]
32. Tse WS, Bond AJ. Relationship between baseline cortisol, social functioning and depression: a mediation analysis. Psychiatry Res 2004; 126:197-201. [ Links ]
33. Weibel L. Methodological guidelines for the use of salivary cortisol as biological marker of stress. Presse Med 2003;32:845-51. [ Links ]
34. Koray M, Dulger O, Ak G, et al. The evaluation of anxiety and salivary cortisol levels in patients with oral lichen planus. Oral Dis 2003;9: 298-301. [ Links ]
35. Rodstrom PO, Jontell M, Hakeberg M, Berggren U, Lindstedt G. Erosive oral lichen planus and salivary cortisol. J Oral Pathol Med 2001; 30: 257-63. [ Links ]
36. McCartan BE, Lamey PJ, Wallace AM. Salivary cortisol and anxiety in recurrent apthous stomatitis. J Oral Pathol Med 1996;25:357-9. [ Links ]
37. Johnson EO, Vlachoyiannopoulos PG, Skopouli FN, Tzioufas AG, Moutsopoulos HM. Hypofunction of the stress axis in Sjögrens syndrome. J Rheumatol 1998;25:1508-14. [ Links ]











 texto en
texto en