My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Medicina Oral, Patología Oral y Cirugía Bucal (Internet)
On-line version ISSN 1698-6946
Med. oral patol. oral cir.bucal (Internet) vol.11 n.6 Nov./Dec. 2006
Oral mucosa symptoms, signs and lesions, in end stage renal disease and non-end stage renal disease diabetic patients
Signos, síntomas y lesiones de la mucosa bucal en diabéticos con y sin insuficiencia renal crónica
Estela de la Rosa García 1, Arnoldo Mondragón Padilla 2, Saray Aranda Romo 3, Martha Alicia Bustamante Ramírez4
(1) MS in Odontology, Professor, Oral Pathology and Medicine Specialization Course. Departamento de Atención a la Salud.
Universidad Autónoma Metropolitana Xochimilco, Mexico, DF, Mexico
(2) Nephrologist. Nephrology service, Hospital General de Zona No 50,
Instituto Mexicano del Seguro Social (IMSS). San Luís Potosí, SLP, Mexico
(3) Oral Pathology and Medicine Specialist. Departamento de Atención a la Salud.
Universidad Autónoma Metropolitana Xochimilco, México, DF, Mexico
(4) Head, Dietology Department. Hospital General de Zona No 50,
Instituto Mexicano del Seguro Social. San Luís Potosí, San Luís Potosí. Mexico
ABSTRACT
Aim: To assess oral signs, symptoms and oral lesions (OL) type and prevalence, in diabetic patients with end stage renal disease (ESRD DM), and compare them with analogous findings in a non-ESRD DM group; analyze the possible association between oral manifestations, as well as with relevant laboratory findings.
Research design. Two adult groups were studied: Group A: ESRD DM on dialysis, and group B: non-ESRD DM (serum creatinine <2.0 mg/dl). Known DM evolution time, dialysis treatment type and duration, and laboratory results were recorded. An oral exam was performed, searching for signs, symptoms and ESRD-associated OL. Associations were analyzed using Chi square, Fishers exact test, and odds ratios (OR) with 95% confidence intervals. Ages, time on dialysis, and laboratory results were compared with Students t test.
Results: 229 individuals were examined, group A 99, and group B 130 pts. Signs and symptoms prevalence was higher in group A: 77.8% vs. 57.6%, (P<0.001), uremic breath (48.5%), unpleasant taste (45.5%) and xerostomia (44.4%) being the most frequent ones. OL were also more prevalent in group A; 65.6% vs. 36.9% (P<0.001). The most frequent OL were dry, fissured lips (28.3%), saburral tongue (18.2%) and candidiasis (17.2%). No difference was found in candidiasis prevalence between groups. Candidiasis was found associated to xerostomia (P<0.05) and smooth tongue (P<0.05) only in group A.
Conclusions. ESRD DM patients had a significantly higher prevalence of signs, symptoms and OLs, as compared to non-ESRD DM pts. The high prevalence of uremic fetor, xerostomia, saburral tongue and candidiasis in group A, could be tried as warning signs on the possibility of non diagnosed advanced renal disease in other diabetic patients.
Key words: End stage renal disease, diabetes, uremic fetor, xerostomia, saburral tongue.
RESUMEN
Objetivos. Conocer el tipo y frecuencia de signos, síntomas y lesiones bucales (LB) en pacientes diabéticos (DM) con insuficiencia renal crónica (IRCT), y compararlos con un grupo de DM sin IRCT. Investigar la posible asociación de las manifestaciones bucales entre sí, y con resultados de laboratorio relevantes.
Diseño del estudio. Fueron dos grupos de adultos: grupo A: DM con IRCT y diálisis, y grupo B: DM sin IRCT (con creatinina sérica <2.0 mg/dl). Se registró tiempo de evolución conocida de la DM, tipo y duración del tratamiento dialítico y resultados de laboratorio. Se realizó un examen bucal registrando signos, síntomas y LB asociadas a IRCT. Las asociaciones se investigaron con χ2, prueba exacta de Fisher y razón de momios (RM) con límites confianza de 95%. Las edades, el tiempo en diálisis y los resultados de laboratorio se compararon con prueba de T de Student.
Resultados. Fueron 229 sujetos; grupo A 99 y grupo B 130. La frecuencia de signos, síntomas fue mayor en el grupo A: 77.8 % vs. 57.6%, (P <0.001); los más frecuentes fueron aliento urémico 48.5%, sabor desagradable 45.5% y xerostomía 44.4%. Las LB también fueron más frecuentes en el grupo A; 65.6% vs. 36.9%, (P<0.001). Las más frecuentes fueron labios secos y fisurados 28.3%, lengua saburral 18.2% y candidosis 17.2%. No se encontró diferencia en la prevalencia de candidosis entre los dos grupos. La candidosis se asoció con xerostomía (P<0.05) y con dorso de lengua liso (P<0.05) solo en el grupo A.
Conclusiones. Los diabéticos con IRCT presentaron un número significativamente mayor de signos, síntomas y LB que los diabéticos sin IRCT. La elevada frecuencia de aliento urémico, xerostomía, lengua saburral y candidosis en el grupo A, podrían probarse como señales de alerta sobre la posibilidad de enfermedad renal avanzada en otros pacientes diabéticos.
Palabras clave: Insuficiencia renal crónica, diabetes, aliento urémico, xerostomía, lengua saburral.
Introduction
End stage renal disease (ESRD) is the final syndrome for several primary renal diseases, and systemic diseases with renal involvement, causing kidney function loss. ESRD manifestations involve virtually every system, in a clinical condition known as uremic syndrome, characterized by a profound alteration of water, electrolyte, and acid-base homeostasis, as well as retention of uremic toxins normally eliminated through urine, especially protein catabolism nitrogen waste products (1). The condition is incompatible with life, unless the patient starts chronic dialysis treatment or kidney transplantation.
ESRD incidence and prevalence are increasing, as shown in consecutive United States Renal Data System (USRDS) annual data reports. All age groups are affected, but ESRD is predominantly an adult disease. ESRD cause was diabetes in 44.8% of incident USA cases in 2003 (2). In that same report, chronic dialysis patients prevalence was 1,496 per million, and median age at dialysis start increased from 52.8 years in 1980, to 62.7 years in 2003, reflecting improved kidney disease medical care (2).
An ESRD prevalence study on Instituto Mexicano del Seguro Social (IMSS)-affiliated adults (>18 yr), estimated 1.142 persons with creatinine clearance levels <15 ml/min per million adult affiliates (3). That level of renal function damage does already, or will soon, need dialysis treatment (4). Another study on IMSS affiliates found diabetes mellitus as the cause of ESRD in 41.1% of incident cases (5). ESRD mortality is increasing in Mexico; being now 9th cause for women and 10th for men (6).
Diabetes mellitus is an important risk factor for ESRD. In Mexico, Amato et al. (3) reported a 10.9% DM prevalence in 18 yr and older individuals, and found 48.6% of affected subjects unaware. Diabetic patients are at risk for acute and chronic complications, including those on the oral cavity (7) such as xerostomia, glossodynia, bacterial, viral, and fungal infections, and periodontal disease (7-9). A 36.6% to 67% association frequency has been reported between diabetes and oral candidiasis (10).
An up to 90% prevalence has been reported in renal disease patients, either on dialysis or with a kidney transplant, for at least one of more than 30 different signs, symptoms, or oral lesions associated in medical literature to uremic syndrome and kidney transplant, including xerostomia, uremic fetor, pale mucosa, uremic stomatitis and candidiasis (11,13).
The aim of this study was to assess signs, symptoms, and oral lesions (OLs) type and prevalence, in a group of ESRD DM patients on dialysis treatment, and compare them with a group of non-ESRD diabetic patients, exploring possible associations among oral lesions, and between OLs and relevant clinical laboratory results.
Material and methods
Observational, comparative, transversal study, performed in two groups of patients: Group A, ESRD DM, formed by consecutive diabetic patients 18 yr and older, both sexes, with diabetic nephropathy – induced ESRD, attending the outpatient nephrology clinic or the peritoneal or hemodialysis units for dialysis treatment follow-up, and Group B, non-ESRD DM, formed by consecutive diabetic patients 18 yr and older, both sexes, attending outpatient family medicine clinic for diabetes mellitus follow-up. Patients with a serum creatinine ≥ 2.0 mg/dl were excluded. The study was carried out at an IMSS general hospital at San Luis Potosi. Demographical data, time from DM diagnosis, dialysis treatment type and duration, clinical laboratory results for blood hemoglobin, fasting glycemia, urea, creatinine and albumin were collected. Hb A1C results were not available. An informed consent was obtained from each patient to participate in the survey.
An examination was performed of all oral mucosa areas, recording ESRD-associated signs, symptoms, and OL absence or presence. Signs and symptoms identification was objectively searched for, and/or reported by patients. A diagnosis of xerostomia was made when a dry or sticky mucosa was found, and when the patient reported a dry mouth; saliva flow was not measured. Uremic fetor was identified when the patient had a urine-odor breath.
OL diagnosis used acknowledged clinical diagnosis criteria for ESRD-associated oral manifestations (11-13), and oral candidiasis was diagnosed by Holmstrup criteria (14).
Uremic stomatitis: irregular-shaped mucosal erythema, covered by grayish pseudomembranes on the lateral borders or inferior aspect of the tongue, occasionally symptomatic (11, 12).
Saburral tongue (ST): Yellowish-white plaque on tongue dorsum, which could not be scraped-off by a blunt instrument. Slightly elongated filiform papillae and bacterial accumulation were found (15). A periodic acid-Schiff (PAS)-stained cytological smear ruled out candidiasis.
Erythematous candidiasis: Dorsal tongue or vestibular mucosal rounded or ovoid-shaped, depapillated, red area. Sub plaque candidiasis: Red area on hard palate, comprising the total or partial prosthesis area, sometimes with a punctated or small papule-filled appearance. Candidiasis was confirmed by gemmating hyphae, found in a PAS-stained cytological smear (14).
Statistical analysis
Descriptive statistics were calculated: frequencies, percentages, means and standard deviations. Inter- and intra-group variable associations were analyzed with Chi square and Fishers exact tests, where applicable, and by odds ratio (OR) with 95% confidence limits (95% CL). Age, diabetes, and dialysis duration were compared with Students t test for unpaired samples with homogenous variances. A P<0.05 value was considered statistically significant.
Results
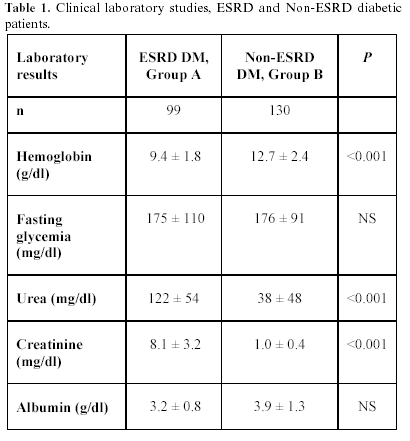
A total of 229 individuals were studied. Group A, 37 men and 62 women, ages 57.9 ± 11.6 (17 to 83) years, and group B, 43 men and 87 women, ages 58.8 ± 11.6 (18 to 77) years (P = NS). Median known DM evolution time: group A, 240 (24 to 408), and group B, 84 (2 to 180) months (P <0.001). Median dialysis treatment time: group A, 8 (1 to 88) months. Table 1 shows clinical laboratory results, revealing, as expected, significant differences in urea, creatinine, and Hb values, corresponding to ESRD and Non-ESRD diagnosis for both groups.
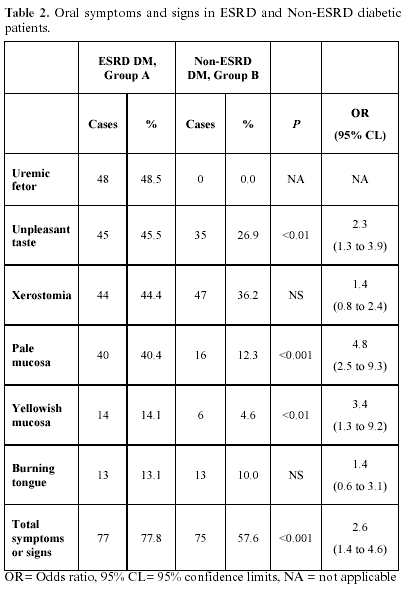
Oral symptoms and signs: Table 2 compares their prevalence in groups A and B, showing odds ratios with 95% CL; Group A had a 77.8% and group B a 57.6% prevalence for at least one symptom or sign (P <0.001).
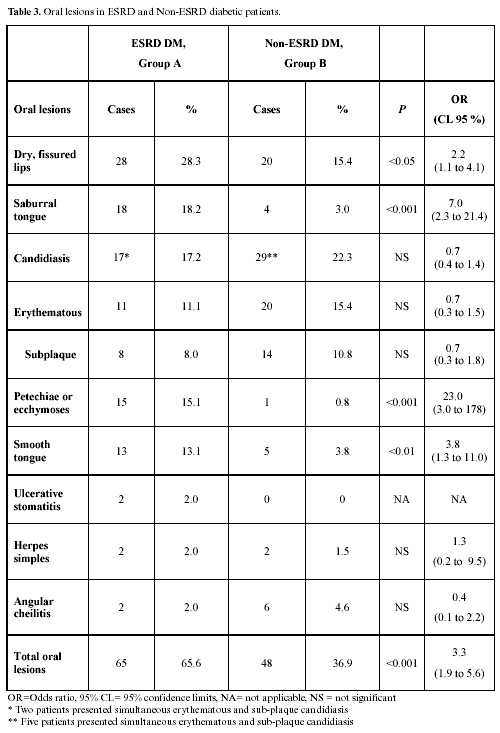
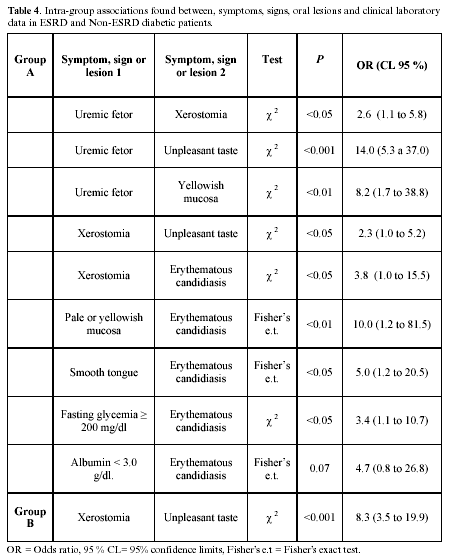
Oral lesions: 97 lesions were found in 65 group A patients; 41 cases had one lesion, 17 cases two, 6 cases three, and 1 patient had four simultaneous lesions; 67 lesions were identified in 48 group B patients; 33 individuals had one lesion, 12 had two, 2 had three lesions, and 1 patient had four simultaneous lesions. Total OL prevalence was greater in group A, 65.6% vs. 36.9% (P<0.001). Table 3 compares OL prevalence in both groups showing odds ratios and 95% CL. There was no difference in candidiasis prevalence. Table 4 shows identified intra-group associations between symptoms, signs, oral lesions and lab results, and odds ratios.
No association was found between smooth tongue and serum albumin or hemoglobin, or between the presence of up to eight signs, symptoms, or OL, and a serum albumin below 3.0 g/dl. No association was found between xerostomia and fasting glycemia or saburral tongue. Microscopic examination of saburral tongue smears revealed abundant bacteria. No cultures were made allowing identification.
Discussion
Symptoms, signs and OL prevalence were significantly higher in ESRD DM patients as compared to Non-ESRD DM. Almost 78% of group A patients had at least one symptom or sign, the most prevalent ones being uremic fetor and unpleasant taste, which were found associated. Uremic fetor was also associated to a yellowish discoloration in the mucosa, and to xerostomia. Uremic fetor occurs as a result of a high urea concentration in saliva, and its ensuing conversion to ammonia (16). additional possible causes are increased phosphate and protein concentrations, and changes in saliva pH, which might explain a metallic or unpleasant taste (1, 12, 17). The 48.5% uremic fetor and 45.5% unpleasant taste found in group A is similar to that reported by Kao et al. (50.0%) (17) and Kho et al. (34.1%) (18) in ESRD hemodialysis patients. Chuang et al. (19) informed a higher prevalence of severe symptoms in diabetics, as compared to non-diabetics in a study of ESRD patients. The higher prevalence of oral manifestations in ESRD DM could be either a worsening of preexisting alterations, caused by longer evolution of DM in group A, or be caused by uremia.
Dry mouth in ESRD patients is a multifactorial phenomenon (17): water restriction, low saliva flow (17, 18, 20, 21), minor salivary glands parenchymal fibrosis and atrophy (21), mouth breathing and medication use (12) being identified factors. The 44.4% found xerostomia prevalence in group A agrees with 32.9% to 68% figures reported in other studies of dialysis patients (17, 18, 21). Xerostomia is also a frequent symptom in the non-ESRD DM patient (7,8), and its prevalence in group B was 36.2%. Minor salivary glands involvement by microvascular disease and neuropathy play an important role in this symptom (7,8,10). Xerostomia in the ESRD DM patient is a risk factor for candidiasis, dental caries, periodontal disease, and bacterial infections, because of the lost protective action of saliva (17,20). Xerostomia is also associated to taste loss (21). A higher prevalence of oral manifestations (19) and gingival calculi (16, 18) has been described in ESRD patients with xerostomia. Chuang et al. (19) reported a possible association between dry mouth and poor glycemic control in hemodialysis patients. Glycemic control was poor for both of our study groups, which probably was a factor in the high prevalence of the symptom.
Mucosa pallor is explained mainly by anemia, a multifactorial complication in the ESRD patient caused by erythropoietin and folic acid deficiencies, inhibited erythropoiesis, shortened erythrocyte life span, hemolysis and hemodialysis complications, among other (1,11,12). Pale mucosa prevalence was significantly higher in group A. The χ2 test could not, however, find an association to blood Hb, perhaps because normal Hb values were missing in group A patients. Pale mucosa has also been associated to malnutrition (11), a reportedly frequent finding (82%) in Mexican dialysis patients, especially diabetic women (22). We were not able to find an association between pale or yellowish mucosa and a serum albumin <3.0 g/dl. The yellowish mucosa discoloration is caused by urocromo pigments (1,11, 12).
Our findings are in agreement to previous reports of ESRD predisposing to oral lesions. The most frequent OL were dry- fissured lips, saburral tongue, and candidiasis. Saburral tongue is an asymptomatic entity. Its prevalence in group A was 18.2%, no association being found to other oral manifestations. Reported prevalences are 12.2% to 47.1% (17-19). Chuang et al. (19) found 47.1% in non-diabetic and 39.5% in diabetic Chinese hemodialysis patients. Saburral tongue is caused by retention of desquamated epithelial cells and dead leucocytes on filiform papillae (15), and by volatile sulfurous compounds produced by anaerobic bacteria on the tongue surface, almost always the same as those found in the subgingival plaque (23). Saburral tongue has also been described as filiform papillae enlargement, with bacteria accumulation due to factors such as a water-restricted diet, low saliva flow, poor oral hygiene, and even the emotional condition of the dialysis patient (15, 24). It has also been reported in transplanted patients: 22.2% in kidney- and 28.3% in liver- transplant patients (25, 26).
The causes of the immunocompromised condition of the dialysis patient are uremia, malnutrition, dysfunctional celular immunity. Immunocompromise increases the risk for opportunistic Candida sp infections. (12). Other known risk factors for candidiasis are xerostomia, low saliva flow, total dental prosthesis, poor oral hygiene, age, diabetes (13, 27). In agreement to other reports (18, 19), candidiasis was found associated to xerostomia in group A, and an almost significant association was also found to a low serum albumin, odds ratio 4.7, 95% CL 0.8 to 27. The association of candidiasis to pale mucosa and smooth tongue could suggest malnutrition predisposing Candida infection. Erythematous candidiasis was the most common type, in most cases occurring on tongue dorsum. The similar prevalence of candidiasis found in groups A and B could mean a stronger association of candidiasis to diabetic, as compared to ESRD condition. Poor glycemic control in both groups could also be a risk factor for candidiasis. We found candidiasis associated to a fasting glycemia above 200 mg/dl in group A, but not in group B. On the other hand, dental prosthesis-associated candidiasis was more prevalent in group B, because edentulous non ESRD DM patients wore a total prosthesis more frequently than edentulous ESRD DM patients. Reported prevalences of oral Candida sp infection in dialysis patients are 5.7 to 37% (28,24), differences probably being caused by diagnostic criteria discrepancy. Klassen and Krasko (29), for instance, reported a 1% central rhomboid glossitis and 12% erythematous patches prevalences in dialysis patients, both probably equivalent to candidiasis. In that same study (29), angular cheilitis was reported in 4% patients. Angular cheilitis has been found associated to Candida sp. infections and anemia (14,27). Its prevalence in group A was 2%. The impact of oral candidosis diagnosis and treatment is to improve oral symptoms and lowering the risk for dissemination to other organs.
Platelet aggregation is altered in uremia (1, 30), which, added to heparin and other anticoagulants used in hemodialysis, predisposes to ecchymosis, petechiae and hemorrhages in the oral cavity (24,30). We found 15.2% cases with ecchymosis and/or petechiae in group A, vs. 0.8% in group B (P<0001). Kho et al. (18) found petechiae and/or ecchymosis in 12.2% of their dialysis patients, and figures as high as 23.3% have been reported in diabetic and non diabetic ESRD patients (17, 19). These lesions were found on oral examination, and had modest clinical relevance.
Oral mucosa ulcerations are yet another class of OLs in dialysis patients, with reported prevalences of 1.2 to 10% (17, 19, 28, 29). Uremic stomatitis, now an uncommon OL because of usually earlier dialysis therapy for ESRD patients, is a localized or generalized burning oral mucosa erythema, with erythematous areas covered by a grayish pseudomembranous exudate leaving, on removal, an intact (type I) or ulcerated (type II) mucosa (12, 13, 31). It has also been described as white plaques on vestibular mucosa and tongue dorsum or belly (31, 32), called hyperparakeratosic uremic stomatitis, often coexisting with Candidiasis. Its etiology is unknown. It has been considered the reaction to a tissular irritant (13, 32) possibly ammonia compounds derived from urea hydrolysis by salivary urease, whenever saliva urea concentration exceeds 180 mg/dl (32). We found two cases, just starting dialysis therapy, with OL matching uremic stomatitis description. Uremia control is the main therapy; 10% hydrogen peroxide gargles (1:1 in water) q.i.d. assist on lesion healing (32).
Conclusions
ESRD DM patients had a significantly higher prevalence of signs, symptoms and oral lesions, as compared to non-ESRD DM patients. This observation agrees with previous reports of ESRD predisposing to oral manifestations in the diabetic patient. Even if an association might be expected between oral manifestations type or number and nutritional status in the diabetic dialysis patient, we were not able to find an association between several oral manifestations and serum albumin, a sensible –but non specific- marker of nutritional status in the diabetic dialysis patient. Oral manifestations were, moreover, barely symptomatic when present, or were probably less of a trouble for the patient as compared to other manifestations of ESRD. Those frequently found conditions of uremic fetor, unpleasant taste, xerostomia, pale or yellowish mucosa, burning tongue, dry- fissured lips, candidiasis, saburral- or smooth tongue in our study group, could be tried as warning signs for undiagnosed kidney disease in other diabetic patients. OL diagnosis and treatment will contribute to improve the quality of life of the difficult ESRD DM patient.
References
1. Skorecki K, Green J, Brenner BM. Chronic renal failure. En: Kasper DL, Braunwald E, Fauci AS, Hauser SL, Longo DL, Jameson JL, eds. Harrison´s Principles of Internal Medicine. New York: McGraw-Hill; 2005. p. 1653-63. [ Links ]
2. United States Renal Data System: Incidence & Prevalence of ESRD. United States Renal Data System 2005 Annual Data Report. Disponible en línea http://www.usrds.org/2005. [ Links ]
3. Amato D, Alvarez AC, Castañeda LR, Rodríguez E, Avila DM, Arreola F, et al. Prevalence of chronic kidney disease in an urban Mexican population. Kidney Int 2005;68:S11-7. [ Links ]
4. K/DOQI Clinical practice guidelines for chronic kidney disease: evaluation, classification and stratification. New Cork: National Kidney Foundation; 2002. p.1-21. [ Links ]
5. Amato MJD, Paniagua SJR. Prevalencia de insuficiencia renal crónica en la población derechohabiente del Instituto Mexicano del Seguro Social. En: García PMC, Reyes MH Viniegra VL eds. Las múltiples facetas de la investigación en salud: Proyectos Estratégicos Del Instituto Mexicano Del Seguro Social. México: D. F. IMSS; 2001. p.153-70. [ Links ]
6. Estadísticas de mortalidad en México: muertes registradas en el año 2003. Salud Pública de México 2005; 47:171-87. [ Links ]
7. Manfredi M, McCullough MJ, Vescovi P, Al-Kaarawi ZM, Porter SR. Update on diabetes mellitus and related oral diseases. Review article. Oral Dis 2004;10:187-200. [ Links ]
8. Arrieta-Blanco JJ, Bartolomé-Villar B, Jiménez-Martinez E, Saavedra-Vallejo P, Arrieta-Blanco FJ. Bucco-dental problems in patients with Diabetes Mellitus (1): Index of plaque and dental caries. Med Oral 2003;8:97-109. [ Links ]
9. Arrieta-Blanco JJ, Bartolomé-Villar B, Jiménez-Martinez E, Saavedra-Vallejo P, Arrieta-Blanco FJ. Bucco-dental problems in patients with Diabetes Mellitus (1): Index of plaque and dental caries. Med Oral 2003;8:233-47. [ Links ]
10. Molina SD, Monroy RE, Arenas R, Fernández RF, Rubalcaba PJ, Fabián SMG. Frecuencia de candidiasis oral en pacientes diabéticos tipo 2 ambulatorios en el Hospital General Manuel Gea González. Estudio clínico micológico. Dermatología Rev Mex 2002;46:3-9. [ Links ]
11. Chronic renal failure and dialysis. En: Little JW, Falace DA, Miller CS, Rhodus Nl eds. Dental management of the medically compromised patient. United States of America: Mosby; 2002. p.147-60. [ Links ]
12. Cohen SG. Enfermedades renales En: Lynch MA, Brightman VJ, Greenberg MS eds. Medicina bucal de Burket. México: McGraw-Hill Interamericana Editores;1996. p. 492-514. [ Links ]
13. Proctor R, Kumar N, Stein A, Moles D, Porter S. Oral and dental aspects of chronic renal failure. J Dent Res 2005;84:199-208 [ Links ]
14. Holmstrup P, Axéll T. Classification and clinical manifestations of oral yeast infections. Acta Odontol Scand 1990; 48:57-9. [ Links ]
15. Morita M, Wang H-L. Association between oral malodor and adult periodontitis: a review. J Clin Periodontol 2001;28:813-9. [ Links ]
16. Epstein SR, Mandel I, Scopp IW. Salivary composition and calculus formation in patients undergoing hemodialysis. J Periodontol 1980; 51:336-8. [ Links ]
17. Kao Ch H, Hsieh JF, Tsai S Ch, Ho YJ, Chang HR. Decreased salivary function in patients with end-stage renal disease requiring hemodialysis. Am J Kidney Dis 2000;36:1110-4. [ Links ]
18. Kho HS, Lee SW, Chung SCh, Kim YK. Oral manifestations and salivary flow rate, pH, and buffer capacity in patients with end-stage renal disease undergoing hemodialysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1999;88:316-9. [ Links ]
19. Chuang SF, Sung JM, Kuo SC, Huang JJ, Lee SY. Oral and dental manifestations in diabetic and nondiabetic uremic patients receiving hemodialysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2005;99:689-95. [ Links ]
20. Kaya M. Cermik TF, Üstun F, Sen S, Berkarda S. Salivary function in patients with chronic renal failure undergoing hemodialysis. Ann Nuclear Med 2002;16:117-20. [ Links ]
21. Postorino M, Catalano C, Martorano C, Cutrupi S, Marino C, Cozzupoli P, et al. Salivary and lacrimal secretion is reduced in patients with ESRD. Am J Kidney Dis 2003;42:722-8. [ Links ]
22. Espinosa A, Cueto MAM, Velazquez AC, Hernández A, Cruz N, Zamora B. et al. Prevalence of malnutrition in Mexican CAPD diabetic and nondiabetic patients. Adv Perit Dial 1996;12:302-6. [ Links ]
23. Van Winkelholff AJ, van der VU, Winkel EG, De Graaff J. Black-pigmented Bacteroides and motile organisms on oral mucosal surfaces in individuals with and without periodontal breakdown. J Periodontal Res 1986;21:434-9. [ Links ]
24. Ziccardi VB, Saini J, Demas PN, Braun TW. Management of the oral and maxillofacial surgery patient with end-stage renal disease. J Oral Maxillofac Surg 1992;50:1207-12. [ Links ]
25. De la Rosa GE, Mondragón PA, Irigoyen CME, Bustamante RMA. Oral lesions in a group of kidney transplant patients. Med Oral Pathol Oral Cir Bucal 2005;10:196-204. [ Links ]
26. Díaz ML, Micó JM, Gargallo J, Baliellas C, Berini L, Gay C. Dental health in liver transplant. Med Oral Patol Oral Cir Bucal 2005;10:66-76. [ Links ]
27. Oksala E. Factors predisposing to oral yeast infections. Acta Odontol Scand1990;48:71-4. [ Links ]
28. Gavaldá C, Bagán JV, Scully C, Silvestre FJ, Milián MA, Jiménez Y. Renal hemodialysis patients: oral, salivary, dental and periodontal findings in 105 adult cases. Oral Dis 1999;5:299-302 [ Links ]
29. Klassen JT, Krasko BM. The dental health status of dialysis patients. J Can Dent Assoc 2002;68:34-8. [ Links ]
30. Kerr A R. Update on renal disease for the dental practitioner. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2001;92:9-16. [ Links ]
31. Ross WF, Salisbury PL. Uremic stomatitis associated with undiagnosed renal failure. Gen Dent 1994;9:410-2. [ Links ]
32. Leão JC, Gueiros LAM, Segundo AVL, Carvalho AT, Barret W, Porter SR. Uremic stomatitis in chronic renal failure. Clinics 2005;60:259-62. [ Links ]
![]() Correspondence:
Correspondence:
Dra. Estela de la Rosa García
Cerro de la Estrella 117-401
Col. Campestre Churubusco
04200 México D.F.
E-mail : delarosa0712@msn.com
Received: 7-01-2006
Accepted: 3-06-2006