My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Medicina Oral, Patología Oral y Cirugía Bucal (Internet)
On-line version ISSN 1698-6946
Med. oral patol. oral cir.bucal (Internet) vol.12 n.1 Jan. 2007
Effects of diabetes on the osseointegration of dental implants
Ana Mellado Valero1, Juan Carlos Ferrer García2,3, Agustín Herrera Ballester 2,3, Carlos Labaig Rueda1
(1) Department of Prosthodontics and Occlusion. School of Dentistry. Valencia University
(2) Diabetes and Endocrinology Unit. Department of Internal Medicine. Valencia University General Hospital Consortium
(3) Department of Medicine. School of Medicine. Valencia University
ABSTRACT
The increased prevalence of diabetes mellitus has become a public health problem. Hyperglycaemia entails a rise in the morbidity and mortality of these patients. Although a direct relationship with periodontal disease has already been shown, little is known about the results of dental implants in diabetics.
The present paper reviews the bibliography linking the effect of diabetes on the osseointegration of implants and the healing of soft tissue. In experimental models of diabetes, a reduced level of bone-implant contact has been shown, and this can be reversed by means of treatment with insulin. Compared with the general population, a higher failure rate is seen in diabetic patients. Most of these occur during the first year of functional loading, seemingly pointing to the microvascular complications of this condition as a possible causal factor. These complications also compromise the healing of soft tissues. It is necessary to take certain special considerations into account for the placement of implants in diabetic patient. A good control of plasma glycaemia, together with other measures, has been shown to improve the percentages of implant survival in these patients.
Key words: Diabetes Mellitus, hyperglycaemia, osseointegration, implant.
RESUMEN
El incremento en la prevalencia de la diabetes mellitus se ha convertido en un problema de salud pública. La hiperglucemia conlleva un aumento en la morbilidad y mortalidad de estos pacientes. Aunque ya se ha demostrado una relación directa con la enfermedad periodontal, poco se conoce sobre el resultado del implante dental en el sujeto diabético.
En el presente trabajo se revisa la bibliografía que relaciona el efecto de la diabetes sobre la oseointegración de los implantes y la cicatrización de los tejidos blandos. En modelos experimentales de diabetes se ha demostrado una reducción en los niveles de contacto hueso-implante, que puede ser revertida mediante tratamiento con insulina. En el paciente diabético, comparado con la población general, se observa un mayor índice de fracaso. La mayoría de ellos se producen durante el primer año de carga funcional, lo que parece señalar a las complicaciones microvasculares de la enfermedad como posible factor causal. Dichas complicaciones comprometen también la cicatrización de los tejidos blandos. Se hace necesario establecer unas consideraciones especiales para la colocación de implantes en el paciente diabético. El buen control de la glucemia plasmática, junto con otras medidas, ha demostrado mejorar los porcentajes de supervivencia de los implantes en estos pacientes.
Palabras clave: Diabetes Mellitus, hiperglucemia, oseointegración, implante.
Introduction
Diabetes Mellitus is a group of metabolic disorders characterized by an increase in plasma glucose levels. This hyperglycaemia is the result of a defect in insulin secretion, insulin action, or both. It is one of the main causes of morbidity and mortality in modern society and has become an alarming public health problem. In the last decade, diabetes affected approximately 140 million individuals and it is expected to affect over 220 million by 2010 and more than 300 in 2025 (1). The prevalence of diabetes mellitus in Spain is estimated at 6.2% for the 30 65 age group and 10% for 30 to 89-year-olds, 90% of whom will be Type 2 diabetics (2).
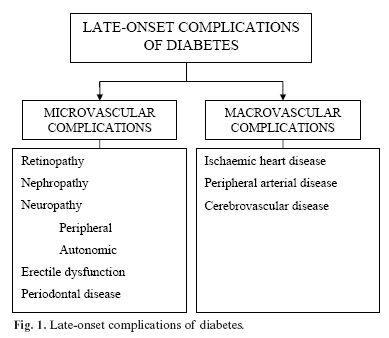
Chronically high levels of plasma glycaemia lead to the onset of chronic vascular complications of this condition, a frequent cause of morbidity and mortality in these patients (Figure 1). The treatment of diabetes aims at achieving optimal metabolic control so as to avoid or delay these complications (3). Over the last few years, special importance has been given to the relationship between diabetes and oral pathologies. Periodontal disease, frequently co-existing with diabetes, is considered to be a further complication of this disease. It affects both patients with type 1 and type 2 diabetes mellitus, and it increases the risk of severe periodontitis by a factor of 3 to 4 times (4).
The impact of diabetes on dental implants has not yet been cleared up. The present article will review the implications of diabetes and glycaemic control for the prognosis and evolution of dental implants, in order to establish, if possible, a series of special considerations for these subjects.
Effect of diabetes on bone
1. Effect of hyperglycaemia
Chronic hyperglycaemia affects different tissue structures, produces an inflammatory effect and, in vitro, has been shown to be a stimulus for bone resorption. Bone loss in diabetes does not seem to depend so much on an increase in osteoclastogenesis as in the reduction in bone formation (5). Hyperglycaemia inhibits osteoblastic differentiation and alters the response of the parathyroid hormone that regulates the metabolism of phosphorus and calcium (6). In addition, it produces a deleterious effect on the bone matrix and its components and also affects adherence, growth and accumulation of extra-cellular matrix (7). Mineral homeostasis, production of osteoid and, in short, bone formation has been shown to be clearly diminished in various experimental models of diabetes (8) (Fig. 2).
2. Differences by type of diabetes
Type 1 diabetes mellitus is an auto-immune disease affecting the beta cells in the pancreas that produce insulin, thus making it necessary to use exogenous insulin to ensure survival and prevent or delay the chronic complications of this illness. Type 2 diabetes mellitus, on the other hand, is a multi-factorial disease resulting from environmental effects on genetically predisposed individuals and is related with obesity, age and a sedentary lifestyle. In these patients, there is a defect in the secretion of insulin together with a greater or lesser degree of insulinopenia. The treatment of type 2 diabetics includes, in stages, measures relating to their diet and lifestyle, oral hypoglycaemic drugs either alone or in combination, and insulin.
In both type 1 and type 2 diabetes, the therapeutic goal focuses on maintaining blood-glucose at normal or near-normal levels. Glycosylated haemoglobin (HbAc1) is used to verify the mean glycaemia of a patient over the last 2 or 3 months, thanks to the correlation between HbAc1 and mean levels of glycaemia shown in Table 1.
Type 1 diabetes produces a reduction in bone mineral density through mechanisms that have not yet been sufficiently clarified; it has been attributed to both a lower formation of bone and also to a greater rate of bone loss (9). This alteration has not been demonstrated in patients with type 2 diabetes and, in some studies, it even seems that there is greater bone mineral density than in the control subjects (10,11). Experimental models of type 2 diabetes have shown a reduction in both bone formation and bone resorption, which might explain this apparently contradictory effect (5).
3. Effects of insulin on bone
Insulin directly stimulates the formation of osteoblastic matrix. In experimental models of diabetes, the normoglycaemia levels obtained by treatment with insulin brought about growth in bone matrix and formation of osteoid similar to control subjects (12). While hyperglycaemia may reduce bone recovery by as much as 40% following circular osteotomies, treatment with insulin normalizes this recovery index, indicating that the deterioration of the bone is strictly related to poor control of diabetes (6).
Effects of diabetes on osseointegration of implants
Although there are articles analyzing the success and failure rates for implants in diabetic patients, only experimental studies with animals have shown the effect of diabetes and insulin therapy on the osseointegration of implants.
1. Results of osseointegration of implants in experimental models of diabetes:
The analysis of the effect of diabetes on implants has revealed an alteration in bone remodelling processes and deficient mineralization, leading to less osseointegration. Some studies have shown that, although the amount of bone formed is similar when comparing diabetes-induced animals with controls, there is a reduction in the bone-implant contact in diabetics (13, 14). One study that analyzed the placement of implants in the femurs of diabetic rodents observed bone neoformation comparable to that of the control group in the region of the periosteum, whereas it was significantly lower in the endosteum and medullar canal, and bone bridges between the endosteum and the implant surface were only observed in a small number of cases (15).
The reduction in the levels of bone-implant contact confirms that diabetes inhibits osseointegration. This situation may be reversed by treating the hyperglycaemia and maintaining near-normal glucose levels (16).
In the light of the articles published, there is a higher probability that the implants will integrate in areas predominated by cortical bone. Nonetheless, further studies are necessary in humans to determine the biological factors affecting osseointegration in diabetic patients.
2. Effect of insulin on bone and osseointegration of implants in experimental models
Various researchers have confirmed that osteopenia associated with diabetes induced in animals can be reversed when treatment with insulin is applied (17).
When implants are placed in the tibia of diabetic rats, a reduction of 50% is observed in the bone formation area and on the contact surface between bone and implant. If insulin is used, the ultra-structural characteristics of the bone-implant interface become similar to those in the control group. These results suggest that metabolic control is essential for osseointegration to take place, as constant hyperglycaemia delays the healing of the bone around the implants (18). Although numerous studies have shown that insulin therapy allows regulation of bone formation around the implants and increases the amount of neoformed bone, it was not possible to equal the bone-implant contact when compared with non-diabetic groups (19).
Implants in patients with diabetes mellitus
Diabetes is currently classified as a relative contraindication for implant treatment. Compared with the general population, a higher failure rate has been seen in diabetic patients with adequate metabolic control (20).
Reviewing the literature published in the last 10 years, the survival rate for implants in diabetic patients ranges between 88.8% and 97.3% one year after placement, and 85.6% to 94.6% in functional terms one year after the prosthesis was inserted. In a retrospective study with 215 implants placed in 40 diabetic patients, 31 failed implants were recorded, 24 of which (11.2%) occurred in the first year of functional loading. This analysis shows a survival rate of 85.6% after 6.5 years of functional use. The results obtained show a higher index of failures during the first year after placement of the prosthesis (21). Another study carried out with 227 implants placed in 34 patients shows a success rate of 94.3% at the time of the second surgery, prior to the insertion of the prosthesis (22). In a meta-analysis with two implant systems placed in edentulous jaws, failure rates of 3.2% were obtained in the initial stages, whereas in the later stages (from 45 months to 9 years), this figure increases to 5.4% (23).
A prospective study with 89 well-controlled type 2 diabetics in whose jaws a total of 178 implants had been placed reveals early failure rates of 2.2% (4 failures), increasing to 7.3% (9 further failures) one year after placement, indicating a survival rate of 92.7% within the first year of functional loading. The 5-year survival rate was 90% (24).
The fact that most failures occur after the second-phase surgery and during the first year of functional loading might indicate microvascular involvement is one of the factors implicated in implant failures in diabetic patients (25, 26).
The percentages of failures in these studies are shown graphically in Figure 3.
The microvascularization alteration associated with diabetes leads to a diminished immune response and a reduction in bone remodelling processes (24, 27). Most of the articles revised conclude that, despite the higher risk of failure in diabetic patients, maintaining adequate blood glucose levels along with other measures improves the implant survival rates in these patients (20, 25).
Special considerations for the placement of implants in diabetic patients
1. Healing and risk of post-operative infection:
The repercussions of diabetes on the healing of soft tissue will depend on the degree of glycaemic control in the peri-operative period and the existence of chronic vascular complications.
Patients with poor metabolic control have their immune defences impaired: granulocytes have altered functionality with modifications in their movement towards the infection site and a deterioration in their microbicide activity, with greater predisposition to infection of the wound. In addition, the high concentration of blood-glucose and in body fluids encourages the growth of mycotic pathogens such as Candida.
The microangiopathy arising as a complication of diabetes may compromise the vascularization of the flap, thus delaying healing and acting as a gateway for the infection of soft tissue (28).
2. Peri-operative measures:
In view of the studies revised, high levels of glucose in plasma have a negative influence on healing and bone remodelling processes.
In order to ensure osseointegration of the implants, understood as the direct bond of the bone with the surface of the implant subjected to functional loading, and to avoid delays in the healing of gum tissue, it is necessary to maintain good glycaemic control before and after surgery. To measure the status of blood-glucose levels in the previous 6 8 weeks, we have to know the HbA1c values. A figure of less than 7% for HbA1c is considered a good level of glycaemic control (the normal value for healthy individuals is 3.5 5.5% depending on the laboratory).
Although there is some controversy over the use of antibiotics in healthy patients, these are recommendable in diabetic patients about to be subjected to implant surgery (22). The antibiotic of choice is amoxicillin (2 gr per os 1 hour previously), as the pathogens most frequently causing post-operative complications following the placement of implants are Streptococci, Gram-positive anaerobes and Gram-negative anaerobes. Clindamycin may also be used (600 mg per os 1 hour previously), azithromycin or clarithromycin (500 mg per os 1 hour previously), and first-generation cephalosporins (cephalexin or cefadroxil: 2 gr per os 1 hour previously) only if the patient has not had any anaphylactic allergic reaction to penicillin (29). In addition to antibiotic prophylaxis, the use of 0.12% chlorhexidine mouthwash has shown a clear benefit by reducing the failure rates from 13.5% to 4.4% in type 2 diabetics, during a follow-up period of 36 months. This same study observed a reduction of 10.5% in the failure rate when antibiotics were administered pre-operatively (20) (Table 2).
Conclusions
There is evidence that hyperglycaemia has a negative influence on bone formation and remodelling and reduces osseointegration of implants. Soft tissue is also affected by the microvascular complications deriving from hyperglycaemia, vascularization of the tissue is compromised, healing is delayed and wounds are more predisposed to infection. This entails an increase in the percentage of failures in the implant treatment of diabetic patients.
The bibliography reviewed recommends good glycaemic control in the peri-operative period in order to improve the survival rates for implants in diabetics. HbA1c figures of less than 7% indicate appropriate glycaemia levels in the preceding 6 8 weeks. Pre-operative antibiotic therapy and the use of 0.12% chlorhexidine mouthwash are recommended as both measures have been shown to reduce the percentage of failures.
Although there is a higher risk of failure in diabetic patients, experimental studies have shown that the optimization of glycaemic control improves the degree of osseointegration in the implants. Nonetheless, it is necessary to extend the number of prospective studies in humans in order to clarify the true impact of diabetes on the prognosis for osseintegration.
References
1. Zimmet P, Shaw J, Alberti KG. Preventing Type 2 Diabetes and the dysmetabolic syndrome in the real world: a realistic view. Diabetes Metab 2003;29:609-18. [ Links ]
2. Goday A. Epidemiología de la Diabetes y sus complicaciones no coronarias. Rev Esp Cardiol 2002;55:657-60.
3. Diabetes Control and Complications Trial Research Group. The effect of intensive treatment on the development and progression of long-term complications in insulin-dependent diabetes mellitus. New Engl J Med 1993;329:977-86.
4. Löe H. Periodontal disease: the sixth complication of diabetes mellitus. Diabetes Care 1993;16:329-34.
5. He H, Liu R, Desta T, Leone C, Gerstenfeld LC, Graves DT. Diabetes causes decreased osteoclastogenesis, reduced bone formation and enhanced apoptosis of osteoblastic cells in bacteria stimulated bone loss. Endocrinology 2003;145:1447-52.
6. Santana RB, Xu L, Babakhanlou C, Amar S, Graves DT, Trackman PC. A role for advanced glycation end products in diminished bone healing in type 1 Diabetes. Diabetes 2003;52:1502-10.
7. Weiss RE, Gora A, Nimni ME. Abnormalities in the biosynthesis of cartilage and bone proteoglycans in experimental diabetes. Diabetes 1981;30:670-7.
8. Nyomba BL, Verhaegue J, Tomaste M, Lissens W, Bouillon RB. Bone mineral homeostasis in spontaneously diabetic BB rats. Abnormal vitamin D metabolism and impaired active intestinal calcium absortion. Endocrinology 1989;124:565-72.
9. Levin M, Boisseau V, Avioli L. Effects of diabetes mellitus on bone mass in juvenile and adult onset-diabetes. N Engl J Med 1976;294:241-5.
10. Krakauer J, McKenna M, Burderer N, Rao D, Whitehouse F, Parfitt A. Bone loss and bone turnover in diabetes. Diabetes 1995;44:775-82.
11. Tuominen J, Impivaara O, Puukka P, Ronnenmaa T. Bone mineral density in patients with type 1 and type 2 diabetes. Diabetes Care 1999;22:1196-200.
12. Locatto ME, Abranzon H, Caferra D, Fernández MC, Alloatti R, Puche RC. Growth and development of bone mass in untreated alloxan diabetic rats. Effects of collagen glycosilation and parathyroid activity on bone turnover. Bone Miner 1993;23:129-44.
13. McCracken M, Lemons JE, Rahemtulla F, Prince CW, Feldman D. Bone response to titanium alloy implants placed in diabetic rats. Int J Oral Maxillofac Implants 2000;15: 345-54.
14. Nevins ML, Karimbux NY, Weber HP, Giannobile WV, Fiorellini JP. Wound healing around endosseous implants in experimental diabetes. Int J Oral Maxillofac Implants 1998;13:620-9.
15. Ottoni CEC, Chopard RP. Histomorphometric evaluation of new bone formation in diabetic rats submitted to insertion of temporary implants. Braz Dent J 2004;15:87-92.
16. Kopman JA, Kim DM, Rahman SS, Arandia JA, Karimbux NY, Fiorellini JP. Modulating the effects of diabetes on osseointegration with aminoguanidine and doxycycline. J Periodontol 2005;76:614-20.
17. Goodman W, Hori M. Diminished bone formation in experimental diabetes. Relationship to osteoid maduration and mineralization. Diabetes 1984;33:825-31.
18. Siqueira JT, Cavalher-Machado SC, Arana-Chavez VE, Sannomiva P. Bone formation around titanium implants in the rat tibia: role of insulin. Implant Dent. 2003;12:242-51.
19. Fiorellini JP, Nevins ML, Norkin A, Weber HP, Karimbux NY. The effect of insulin therapy on osseointegration in a diabetic rat model. Clin Oral Implants Res 1999;10:362-8.
20. Morris HF, Ochi S, Winkler S. Implant survival in patients with type 2 diabetes: placement to 36 months. Ann Periodontol 2000;5:157-65.
21. Fiorellini JP, Chen PK, Nevins M, Nevins ML. A retrospective study of dental implants in diabetic patients. Int J Periodontics Restorative Dent 2000;20:366-73.
22. Balshi TJ, Wolfinger GJ. Dental implants in the diabetic patient: a retrospective study. Implant Dent 1999;8:355-9.
23. Esposito M, Hirsch JM, Lekholm U, Thompson P. Failure paterns of four osseointegrated oral implant systems. J Mat Sci Mater Med 1997;8:843-7.
24. Olson JW, Shernoff AF, Tarlow JL, Colwell JA, Scheetz JP, Bingham SF. Dental endosseous implant assessments in a type 2 diabetic population: a prospective study. Int J Oral Maxillofac Implants 2000;15:811-8.
25. Farzad P, Andersson L, Nyberg J. Dental implant treatment in diabetic patients. Implant Dent 2002;11:262-7.
26. Peled M, Ardekian L, Tagger-Green N, Gutmacher Z, Matchei EF. Dental implants in patients with type 2 diabetes mellitus: a clinical study. Implant Dent 2003;12:116-22.
27. Beiker T, Flemmig T. Implants in the medically compromised patient. Crit Rev Oral Biol Med 2003;14:305-16.
28. Mealey BL, Moritz AJ. Influencias hormonales: efectos de la diabetes mellitus y las hormonas sexuales esteroideas endógenas femeninas en el periodonto. Periodontology 2000 2004;7:59-81.
29. Beikler T, Flemming TF. Antimicrobials in implant dentistry. In: Antibiotic and antimicrobial use in dental practice. Newman M, van Winkelhoff A, editors. Chicago: Quintessence; 2001. p. 195-211.
![]() Correspondence:
Correspondence:
Dr. Juan Carlos Ferrer García
Unidad de Diabetes. Servicio de Medicina Interna.
Consorcio Hospital General Universitario de Valencia.
Av. Tres Cruces s/n
46014 Valencia
E-mail: ferrer_juagar@gva.es
Received: 4-06-2006
Accepted: 1-10-2006