Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Medicina Oral, Patología Oral y Cirugía Bucal (Internet)
versión On-line ISSN 1698-6946
Med. oral patol. oral cir.bucal (Internet) vol.12 no.1 ene. 2007
Control of Freys syndrome in patients treated with botulinum toxin type A
Kuauhyama Luna Ortiz 1, Mario Rascon Ortiz 1, Jesús A. Sansón Riofrio 1,
Verónica Villavicencio Valencia 1, Adalberto Mosqueda Taylor 2
(1) MD, Deparment of Head and Neck Surgery at the Instituto Nacional de Cancerologia. Tlalpan. México
(2) DDS MSc, Departamento de Atención a la Salud, Universidad Autónoma Metropolitana Xochimilco. México, D.F. México
ABSTRACT
Aim: To identify the severity of Freys syndrome and its response to botulinum toxin type A. Methods: Minor test was performed in all cases to assess the extent of the affected area, using the contralateral side as control. Severity was assessed according to the proposal of Luna-Ortiz et al. Response was evaluated after 3 and 6 months, and was compared with the basal data. Results: Freys syndrome was documented in 38 patients, but only 23 cases accepted the botulinum toxin type A treatment. Severity was moderate in 8 (35%) and severe in 15 (65%) cases. Mean applied dose was 1.41 MU/cm2 in 21 patients (91%), whereas one patient was treated with 10 MU for a 0.8 cm2 affected area (12.5 MU/cm2) and another patient with 10 MU for a 0.5 cm2 affected area (20 MU/cm2) due to severity of their symptomatology. Average affected area at the beginning was 14.2 cm2, while after 3 and 6 months of treatment it was 4.1 cm2 and 4.4 cm2 respectively (p<0.001). The two patients that received higher doses of botulinum toxin A had complete response. Complete response was observed in 13 patients (56.5%) at 3 months, but in only nine (39%) this lack of symptomatology persisted at 6 months. In three cases (13%) no response was obtained at 3 months, and the application of an additional dose of botulinum toxin type A produced no response in two of them after 6 months. Comparison of the severity score of the average basal value vs. that obtained at 3 and 6 months revealed a significant difference (p< 0.05); however, no statistically significant difference was found when comparing outcome at 3 vs. 6 months. There were no statistically significant differences using the independent samples test when comparing outcome after treatment in relation to gender, type of surgery, or use of postoperative radiation therapy (p>0.05). In conclusion, botulinum toxin A remains as the treatment of choice for Freys syndrome.
Key words: Botulinum toxin type A, Frey syndrome, gustatory sweating, parotid gland.
Introduction
Gustatory sweating is caused by the disruption of the auriculotemporal nerve branches, followed by an aberrant regeneration resulting in a parasympathetic reinervation with sympathetic receptors. This alteration is known as Freys syndrome (1) based on its first description made by the Polish neurologist Lucja Frey, with an incidence of 5 to 100% in patients subjected to parotidectomy and a mean incidence estimated in 66% (2,3). In a previous study we found an incidence of 36% in a Mexican population (4).
Several treatments have been proposed to avoid this complication, including interpositional barriers by means of dermis grafts (5,6), acellular human dermal matrix grafts (7), synthetic implants (8), interposition of fascia or muscle flaps (9-15), section and ligature of the auriculotemporal nerve (16), as well as other methods aimed at reducing its intensity by means of topical application of anticholinergic agents (17,18) and, recently, with the injection of botulinum toxin type A (19,20).
The present study was aimed at identifying the severity of Freys syndrome and its response to botulinum toxin type A in patients treated at a cancer center in Mexico City.
Material and Methods
This observational study was carried out in patients that had a minimal time of 6 months since they were submitted to partial or total parotidectomy and were under periodic control at the Head and Neck Department at the Instituto Nacional de Cancerologia in Mexico City, regardless of age or age, with or without antecedents of medical or surgical treatment to prevent or to reduce the incidence and/or severity of the symptomatology caused by Freys syndrome.
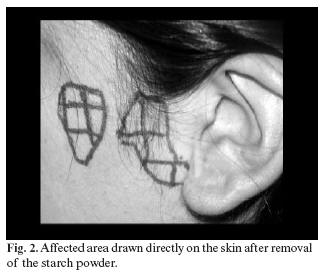
In all cases the starch-iodine Minor (21) test was performed in order to assess the existence and extension of the affected area (Figure 1), using the contralateral side as control in all cases. The affected area (Figure 2) was drawn on a transparent acetate sheet and extension was expressed in square centimeters (cm2) (Figure 3).
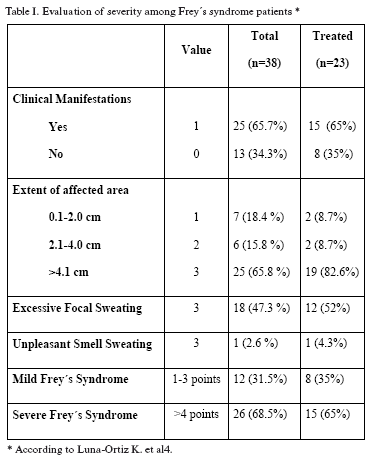
The degree of severity was assessed in each patient according to the proposal of Luna-Ortiz et al.(4), which includes four items as described in Table I. All patients who accepted treatment signed an informed consent, and each of them received intradermally botulinum toxin type A (Botox, Allergan 100 mouse units (MU)/4 ml 0.9% saline solution, Mexico City, Mexico) applied homogeneously on the previously marked affected area. Response to treatment was evaluated through the Minor test and the degree of severity at 3 and 6 months after application of the botulinum toxin type A, and the corresponding figures were compared with the basal ones in each case. Results were assessed by means of Students t and Chi square tests, and, in some instances, by means of the independent samples test.
Results
There were a total of one hundred patients (36 men and 64 women) that were evaluated after partial or total parotidectomy; of these, ninety-seven had been surgically intervened due to parotid tumors and 3 because of irreversible chronic inflammatory processes. Freys syndrome was documented in 38 cases, with an average age of 46.7 years (range, 18 to 70 years). There were 25 women (66%) and 13 men (34%). Of these, sixteen patients (42%) had been subjected to total parotidectomy and 22 (58%) to partial parotidectomy. Seven cases (18.4%) received adjuvant therapy as part of their oncological treatment. The average affected area was 10.32 cm2(range: 1 to 30 cm2). Severity in this group of patients was moderate in 12 (31.5%) and severe in 26 (68.5%), as shown in Table I. Contralateral side was negative in all patients.
Only 23 (8 men and 15 women) of the 38 patients accepted the botulinum toxin type A treatment. Severity in these cases was moderate in 8 (35%) and severe in 15 (65%). Mean applied dose was 1.41 MU/cm2 of the affected skin area in 21 patients (91%), whereas one female patient was treated with 10 MU for a 0.8 cm2 affected area (12.5 MU/cm2) and one male patient was treated with 10 MU for a 0.5 cm2 affected area (20 MU/cm2) due to the severity of their symptomatology.
The average affected area at the beginning of the study was of 14.2 cmv, which ranged from 0.5 to 29.8cm2 (15.1 for men and 13.81 in women). The mean affected area after 3 months of treatment was 4.1 cm2 (5.1 cm2 for men and 3.5 cm2 for women); however 3 patients (13%) required a second application of the botulinum toxin due to lack of response to the initial treatment. The average affected area at 6 months was 4.4 cm2 (7.48 cm2 for men and 2.75 cm2 for women). The two patients that received higher doses of botulinum toxin A than the rest of the group had complete response.
Table II depicts the affected area for each patient at the beginning and at 3 and 6 months of treatment, revealing a response of 79.3% at 3 months and 73.8% at 6 months. Comparisons of the extent of the affected area at baseline with that found at 3 and 6 months revealed a statistically significant difference (p<0.001) between the initial figure and those recorded at 3 and 6 months; however, comparison between 3 and 6 months revealed no significant difference (p=0.726).
In three cases (13%) no response was obtained at 3 months, and the application of an additional dose of botulinum toxin type A produced no response in two of them after 6 months. Comparison of the severity score of the average basal value vs. that obtained at 3 and 6 months revealed a significant difference (p< 0.05); however, no statistically significant difference was found when comparing outcome at 3 vs. 6 months. There were no statistically significant differences using the independent samples test when comparing outcome after treatment in relation to gender, type of surgery, or use of postoperative radiation therapy (p>0.05).
Discussion
The pathogenesis of Freys syndrome is not fully understood. The most frequent hypothesis relates this syndrome to a misdirected regeneration of postganglionic nerve fibers of the auriculotemporal and greater auricular nerve. After nerve injury, these nerve fibers connect to injured postaganglionic sympathetic nerve fibers innervating sweat glands and small skin vessels, producing sweating under gustatory stimuli ("gustatory sweating"). It has been suggested that the probability one individual has for developing this syndrome could be related to the amount of tissue that is removed during parotidectomy (22); however, this seems quite unlikely since although this syndrome has been reported mainly associated to the parotid, it has also been reported in association with trigeminal nerve infection by herpes virus, post-parotiditis, secondary to condylar fractures, after traumatisms with forceps, and after surgery for meningiomas located in the pontocerebellar angle, in which parotid tissue is not removed (23). The present study revealed no difference regarding its presence in patients that were subjected to superficial or total parotidectomy.
According to the diverse series, the incidence of post-parotidectomy Freys syndrome has been reported in a mean of 66%. However, this figure varies widely according to the criteria employed to diagnose this condition. The most frequently used diagnostic criterion is the objective one, by means of the starch-iodine Minor test (21). We found a 38% frequency of post-parotidectomy Frey syndrome when diagnosed with the Minor test; however, this incidence was lower when diagnosis was based solely on clinical criteria (26%). This phenomenon has been previously reported (4), suggesting that there is an asymptomatic presentation of this disorder, in which clinical manifestations are so mild that the patient is unable to perceive them. Another possibility that could explain this low incidence is that the physician does not detect it, either because he/she is unaware of the disorder or considers it so uncommon that pays no attention to it, or perhaps, during examination, questions only address the presence of symptoms. This situation, as has been proven in questionnaires related to epidemiological studies for validation, leads to low detection sensitivity and specificity (24). Therefore, we recommend that every patient treated by means of partial or total parotidectomy be periodically subjected to a complete evaluation, including the Minor test.
The average affected area during basal measurement in this study was 14.2 cm2 (range, 0.5 to 29.8 cm2 ), which seems lower than those reported in other series, where it ranges from 25 to 175 cm2 (25), 16 to 81 cm2 (26), and 36 to 80 cm2 (22); however, no particular explanation seems to exist for this difference.
The use of botulinum toxin type A for the treatment of Frey´s syndrome was introduced by Drobik and Laskawi (19); the dose used in that study was of 0.5 MU; however, the optimal dose has not been establish yet, as it has been applied in diverse studies from 0.5 MU to 5 MU (19,23,27,28). Likewise, no relation dose-area has been established yet, since schemes of 0.5 MU/4 cm2 (19), 2.5 MU/cm2, (26,29), 1 MU/cm2, , and 1 to 2 MU/2.25 cm2 (25) have been used. One explanation of this diversity may be that severity of Frey syndrome has not been clearly defined in all series. Recently, Luna-Ortiz et al published a series of cases and proposed an objective method for evaluating severity, which has been used in the present study (4). In our series we used a mean dose of 1.41 MU/cm2 of the affected skin area in 21 (91%) patients, although one female patient was treated with 10 MU for an affected area of 0.8 cm2 (12.5 MU/cm2) and one male patient with 10 MU for an affected area of 0.5 cm2 (20 MU/cm2) due to the severity of their symptomatology. The observed overall response was of 79.2% at 3 months and of 73.8% at 6 months. Three patients did not respond initially and only one of them responded after the second application. Thirteen (56.5%) patients had a complete response at 3 months, but only 9 of them (39%) persisted with complete response at 6 months of follow up, which reveals a clear tendency to recurrence, as has been shown in another study (25). However, severity downgrading of the symptomatology is clearly diminished when comparing the basal measurement vs. that obtained at 3 months in 14 (60.8%) patients and in 15 (65%) at 6 months.
Several treatment have been used to prevent Freys syndrome, such as placement of interpositional barriers, going from dermal grafts (5,6), acellular human dermal matrix grafts (7), synthetic implants (8), interposition of fascia or muscle flaps (9-15), or even section or ligature of the auriculotemporal nerve (16); however, none of these methods prevents this complication in 100% of cases, which means that another mechanism might be involved besides the anomalous neural regeneration. Another disadvantage of the preventive measures is the over-treating of 62% of the patients subjected to parotidectomy that do not develop this syndrome (4); besides, a large number of salivary fistulas (57%) has been observed after some of these therapeutic modalities, especially when using synthetic materials (8).
Conclusion
Incidence of Freys syndrome in our population is less than 40%, with important differences regarding the extent of the affected area as compared with that reported for Anglo-Saxon patients, who present higher values. The 1.41 MU/cm2 dose used in this study demonstrated a 79% response; however, two patients did not respond at all. Thus far, botulinum toxin A remains as the treatment of choice for the management of Freys syndrome.
References
1. Dunbar EM, Singer TW, Singer K, Knight H, Lanska D, Okun MS. Understanding gustatory sweating. What have we learned from Lucja Frey and her predecessors?. Clin Auton Res 2002;12:179-84. [ Links ]
2. Laage-Hellman JE. Gustatory sweating and flushing after conservative parotidectomy. Acta Otolaryngol 1957;48:85-9.
3. Laccourreye O, Laccourreye H, Couchoix R, Jouffre V, Menard M, Brasnu D. Total conservative parotidectomy for primary benign pleomorphic adenoma of the parotid gland: a 25 years experience with 229 patients. Laryngoscope 1994;104:1487-94.
4. Luna-Ortiz K, Sansón-RioFrio JA, Mosqueda-Taylor A. Frey syndrome. A proposal for evaluating severity. Oral Oncol 2004;40:501-5.
5. Cassady CL. A new cencept in the treatment of Freys syndrome: the use of interpositional dermal grafts. An experimental study in dogs. Laryngoscope 1977;87:962-74.
6. Harada T, Inoue T, Hrashina T, Hatako M, Ueda K. Dermis-fat graft after parotidectomy to prevent Freys syndrome and the concave deformity. Ann Plast Surg 1993;31:450-2.
7. Sinha UK, Saadat D, Doherty CM, Rice DH. Use of AlloDerm implant to prevent Frey syndrome after parotidectomy. Arch Facial Plast Surg 2003;5:109-12.
8. Dulguerov P, Quinodoz D, Cosendai G, Piletta P, Marchal F, Lehmann W. Prevention of Frey syndrome during parotidectomy. Arch Otolaryngol Head Neck Surg 1999;125:833-9.
9. Kornblut AD, Westphal P, Miehlke A. The effectiveness of a sternomastoid muscle flap in preventing post-parotidectomy occurrence of the Frey syndrome. Acta Otolaryngol 1974;77:368-73.
10. Casler JD, Conley J. Sternocleidomastoid muscle transfer and superficial musculoaponeurotic system plication in the prevention of Frey syndrome. Laryngoscope 1991;101:95-100.
11. Rubinstein RY, Rosen A, Leeman D. Frey syndrome:treatment with temporoparietal fascia flap interposition. Arch Otolaryngol Head Neck Surg 1999;125:808-11.
12. Allison GR, Rappaport I. Prevention of Freys syndrome with superficial musculoaponeurotic system interposition. Am J Surg 1993;166:407-10.
13. Ahmed OA, Kolhe PS. Prevention of Freys syndrome and volumen deficit after parotidectomy using the superficial temporal artery fascial flap. Br J Plat Surg 1999;52:256-60.
14. Cesteleyn L, Helman J, King S, Van de Vyvere G. Temporoparietal fascia flaps and superficial musculoaponeurotic system plication in parotid surgery reduces Freys syndrome. J Oral Maxillofac Surg 2002;60:1284-97.
15. Kim SY, Mathog RH. Platysma muscle-cervical fascia-sternocleidomastoid muscle (PCS) flap for parotidectomy. Head Neck 1999;21:428-33.
16. Roark DT, Sessions RB, Alford BR. Freys syndrome a technical remedy. Ann Otol Rhinol Laryngol 1975;84:435-40.
17. May JS, McGuirt WF. Freys syndrome:treatment with topical glycopyrrolate. Head Neck 1989;11:85-9.
18. Laccourreye O, Bonan B, Laccourreye H. Treatment of Freys syndrome with topical 2% diphemanil methylsulfate (Pranta®): A double-blind evaluation of 15 patients. Laryngoscope 1990;100:651-3.
19. Drobik C, Laskawi R. Freys syndrome: treatment with botulinum toxin. Acta Otolaryngol (Stockh) 1995;115:459-61.
20. Drobik C, Laskawwi R, Schwab S. Die Therapie des Frey-Syndroms mit Botulinumtoxin A. Erfahrungen mit einer neuen. Behandlungsmethode. HNO 1995;43:644-8.
21. Minor V. Ein neues Verfahren zu der klinisschen Untersuchung der Schweissabsonderung. Dtsch Z Nervenheilkd 1927;101:302-6.
22. Beerens AJF, Snow GB. Botulinum toxin A in the treatment of patients with Frey syndrome. Br J Surg 2002;89:116-9.
23. Laccourreye O, Muscatello L, Gutierrez-Fonseca R, Seckin S, Brasnu D, Bonan B. Syndrome de Frey severe post-parotidectomie: traitement par la neuro-toxine botulique de type-A. Ann Otolaryngol Chur Cervicofac 1999;116:137-42.
24. Lilienfeld AM, Graham A. Validity of determining circumcision status by questionnaire as related to epidemiological studies of cancer of the cervix. J Nat Cancer Inst 1958;21:713-20.
25. Laccourreye O, Akl E, Gutierrez-Fonseca R, Gracia D, Brasnu D, Bonan B. Recurrent gustatory sweating (Frey Síndrome) after intracutaneous injection of botulinum toxin type A: incidence, management and outcome. Arch Otolaryngol Head Neck Surg 1999;125:283-6.
26. Eckard A, Kuettner C. Treatment of gustatory sweating (Frey`s syndrome) with botulinum toxin A. Head Neck 2003;25:624-8.
27. Naumann M, Zellner M, Toyka KV. Treatment of gustatory sweating with botulinum toxin. Ann Neurol 1997;42:973-5.
28. Dulgerov P, Quinodoz D, Cosendai G, Piletta P, Lehmann W. Frey syndrome treatment with botulinum toxin. Oto-Laryngol Head Neck Surg 2000;122:821-7.
29. Bjerkhoel A, Trobbe O. Frey`s syndrome: treatment with botulinum toxin. J Laryngol Otol 1997;111:839-44.
![]() Correspondence:
Correspondence:
Dr. Kuauhyama Luna-Ortiz, MD.
Department of Head & Neck Surgery
Instituto Nacional de Cancerologia
Av. San Fernando # 22, Tlalpan
México D.F. 14080
E-mail:kuauhyama@starmedia.com
Received: 31-05-2006
Accepted: 25-07-2006