Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Medicina Oral, Patología Oral y Cirugía Bucal (Internet)
versión On-line ISSN 1698-6946
Med. oral patol. oral cir.bucal (Internet) vol.12 no.2 mar. 2007
Myoepithelial cells are the main component in pleomorphic adenomas?
Santa Ponce Bravo, Constantino Ledesma Montes, Uriel López Becerril, Israel Morales Sánchez
Laboratorio de Patología Clínica. División de Estudios de Posgrado e Investigación. Facultad de Odontología, UNAM. México, D.F. MÉXICO
ABSTRACT
Objective: The aim of this study was to quantify by immunohistochemistry the number of myoepithelial cells (MyECs) in pleomorphic adenomas (PAs).
Material and methods: We retrieved the paraffin cubes of 27 PAs, new slides were done and they were stained with anti-S100 protein antibody. The amount of S-100 protein positive cells was quantified, their morphology was recorded and comparison among MyEC number with age, gender and involved gland were also done.
Results: With S-100 protein, MyECs in normal salivary gland tissue were seen surrounding the ductual structures only. In the analysed PAs a mean of 27.4% of the neoplastic cells were positive to the antibody. With the exception of one PA, in all the analysed cases the plasmacytoid cells were the most commonly identified cells (48,6%).
Conclusions: Results of this study suggest that MyECs do not constitute the main cellular component of the neoplastic compartment in PAs and corroborate the previously reported evidence by different au-thors, who studying the PAs suggested that MyECs does not comprise the main cellular neoplastic component of these entities.
Key words: Pleomorphic adenomas, salivary gland tumors, myoepithelial cells, S-100 protein.
RESUMEN
Objetivo: El objetivo de este estudio fue cuantificar por medio de inmunohistoquí-mica el número de células mioepiteliales (CMs) en adenomas pleomorfos (APs).
Material y Métodos: Se recuperaron los cubos de 27 APs y se hicieron nuevas laminillas, las que se tiñeron con un anticuerpo anti-proteína S-100, se contó el número de células S-100 positivas, se registró su morfología y se hicieron comparaciones del número de CMs tomando en cuenta el sexo, edad y glándula de origen.
Resultados: Se observó que en el tejido glandular normal, las CMs solo se observaron alrededor de las estructuras ductuales. En los APs analizados se encontró que en promedio, solamente el 27,4% de las células neoplásicas fueron positivas a este anticuerpo. Con excepción de un AP, en todos los casos analizados las células plasmocitoides fueron las células más comúnmente encontradas (48,6%).
Conclusiones: Los resultados de este estudio sugieren que las CMs no forman el componente celular principal del compartimiento neoplásico de los APs y confirman las evidencias encontradas desde hace varios años, por diferentes autores, quienes estudiando los APs, sugirieron que las CMs no forman la mayor parte de las células neoplásicas en estas entidades.
Palabras clave: Adenoma pleomorfo, tumores de glándulas salivales, células mioepiteliales, proteína S-100.
Introduction
Pleomorphic adenoma (PA) is the most common epithelial neoplasm arising in the salivary gland tissue. Its more frequent extraoral location is parotid and intraorally, palate is the most common place. Among sexes, PA predominates in males (1-5). Differences amongst age, gender and location have been reported (2-5).
PA is characterized by its polymorphic microscopic appearance and several cellu-lar structures have been identified. This polymorphic appearance consists in that the main cellular components of this neoplasm (ductual and myoepithelial cells) are arranged forming different structures: ducts, solid groups or sheets, hyaline, mixocondroid and bone-like tissue as well as keratin pearls and epidermoid metaplastic zones are also seen. Frequently, myoepithelial cells (MyECs) display different shapes: stellate, polygonal, plasmacytoid, fusiform and round or oval.
MyECs have been considered as the main cells composing the neoplastic cell compartment in the PAs (6-9), but this fact was challenged when we carefully analysed the minor results published by several authors (10-14). In these articles, the authors mentioned that PA cells related as MyECs did not show enough features to be consid-ered as such. Reading of these comments let us to think that MyECs are not the most common cells in PA. Also, we made an extensive review of the literature searching on works related to accurately confirm our assumption, but we were not able to find any work dealing on quantification of MyECs in PAs.
The aim of this study was to quantitate the MyEC numbers in different areas of PA cases.
Material and methods
27 PAs from the files of our Oral Pathology Laboratory located in the Facultad de Odontología, UNAM (Mexico City) were retrieved and their clinical features analysed, microscopic slides were reevaluated according to the parameters of the 2005 WHO Histological Classification of Tumours of Salivary Glands (1). Clinical data assessed were age, gender and location of the tumours. New 5µ paraffin embedded slides were done and all slides received previous treatment with 0,1% trypsin solution in oven at 35º C for 5 minutes. For immunohistochemical staining with the anti-S-100 protein MoAb (Dako) the slides were treated with citrate buffer during three min in a microwave oven at 600W, washings were made with PBS-Triton, the streptavidin-biotin method was employed and the slides were counterstained with Harris haematoxylin. Negative controls omitting the antibody were done. Positivity was matched: 0= negative; 1= slight; 2= moderate; 3= strong and 4= very strong. Control slides from normal parotid salivary glands were used and immunostained as above.
For the microscopic review, a Carl Zeiss microscope was employed by two experienced Oral Pathologists, five randomly selected 40X microscopic fields were analysed and all the cells contained in the area of an ocular graticule were counted. S-100 protein positive cells included were: fusiform, plasmocytoid, chondroid, myxoid and epithelial-like. A previous calibration of the examiners was performed, inter-examiners correlation was 94% and intra-examiner correlation was 95%. Review of the stained slides was double blind and a mean of the lectures obtained by both examiners was considered correct. MyEC counts are expressed as number of cells per 40X field.
Results
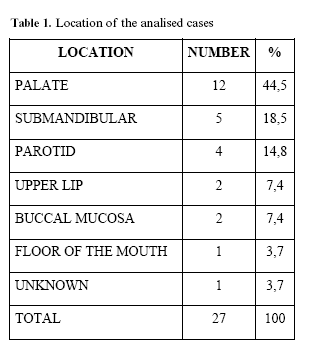
From the 27 patients there were 20 females and 7 males. Patients age was between 14 and 72 years with a mean age of 35,1 years (in three cases age was not specified). Location of the tumours was more frequent in palate and submandibular gland (Table 1). Of the 27 PA cases, 40 slides were reviewed.
All the reviewed cases showed the typical features of AP as it was in the WHO guidelines (1). All the tumours were formed by numerous ductiform structures, myoepithelial cells showed different shapes forming solid structures, chondroid and myxoid tissue, mucoid or diffusely arranged with scarce stromal tissue (Fig. 1).
Positivity to S-100 protein antibody in the control normal salivary gland tissues was found in the myoepithelial cells surrounding the ductual structures only.
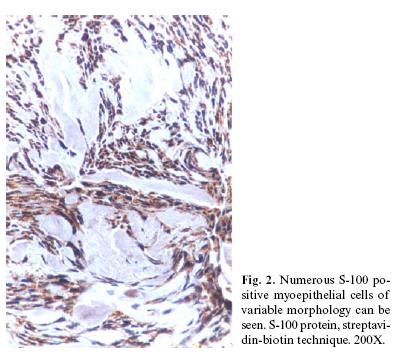
PA cases showed positivity in the cells located around the pseudoductual struc-tures and in the fusiform, plasmocytoid, myxoid, chondroid and epithelial-like cells (Fig. 2 and 3). Despite our efforts to get positivity in the slides from four cases, they showed no positive immunostaining.
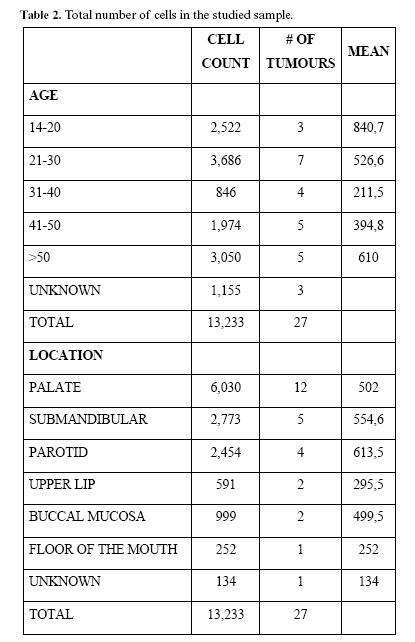
In the analysed slides, 13.233 neoplastic cells (mean= 575 cells/tumour) were quantified. Number of positive cells per microscopic field (40X) varied from 12 cells in a predominately chondromyxoid tumour to 617 cells in the more cellular tumours.
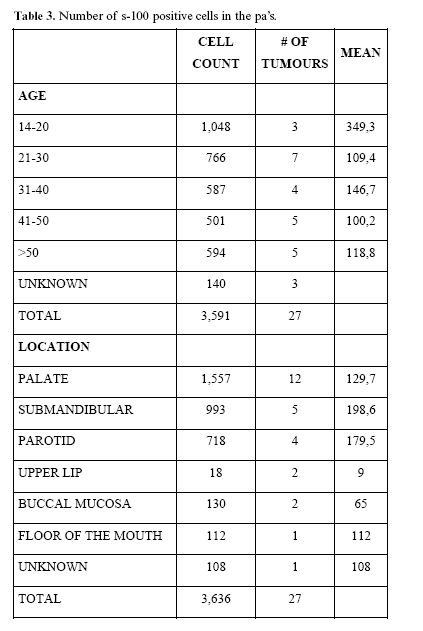
Positivity varied from 3% to 80% of the total neoplastic cell number. Of the 13.233 neoplastic cells, 3.636 (27,4%) were S-100 protein positive cells. Plasmacytoid cells were the more common S-100 positive cells; they were 1.769 cells (13,4%) of the total neoplastic cells and comprised 48,6% of the S-100 positive cells. Chondromyxoid cells found in this study were 942 (7,1%) of the total cell quantitation and 25,9% of the S-100 positive cells. Fusiform cells were 640 comprising 4,8% of the total cell count and 17,6% of the S-100 positive cells. S-100 positive cells grouped in solid areas were the less commonly found cells; they were 285 cells and comprised 2,1% of the total cellular count and 7,9% of the S-100 positive cells.
Our total cell quantitation (Table 2) showed that PAs were more cellular in 20 years or younger patients (mean= 840,7 cells/tumour), followed by patients older than 50 years (mean= 610 cells/tumour). PAs from women were more cellular than those from men. In men tumours there were counted 3.102 cells and a mean of 443,1 cells/tumour was found. In women 10.131 cells were found with a mean of 506,5 cells/tumour. Tumours located in parotid were the more cellular APs (mean= 613,5 cells/tumour), followed by those located in submandibular gland (mean= 554,6 cells/tumour). Other cell counts by age and location are also shown in table 2.
S-100 protein positive cells were more frequently found in males (mean= 147,6 cells/tumour) followed by female tumours (mean= 130,6 cells/tumour). Also, these cells were more numerous in 20 years or younger patients (mean= 349,3 cells/tumour), followed by tumours in patients between 31 to 40 years (mean= 146,7 cells/tumour). Tumours located in submandibular glands were the more cellular APs (mean= 198,6 cells/tumour), followed by those located in parotid glands (mean= 179,5 cells/tumour). All S-100 positive cell counts by age and location are included in table 3.
In tumours from labial glands, only 18 S-100 protein positive cells were found (3,04%) and all of them were of the fusiform type. However, in the PAs located in glands of the buccal mucosa 999 neoplastic cells were quantified, of them, 130 were S-100 positive (13%) and plasmacytoid cells predominate (42,3%). In tumours located in palate 6.030 cells were counted, of them, 1.557 were S-100 protein positive cells (25,8%). The most common MyEC identified was the plasmacytoid cell (61,2%) also. Those PAs situated in parotid glands contained 2.454 neoplastic cells, of them, 718 were S-100 protein positive (29,26%) and also, the plasmacytoid cell was the more commonly identified MyEC (36,1%). From the PAs located in the floor of the mouth 252 cells were counted, of them, 112 were S-100 protein positive (44,4%) and unexpectedly epithelial-like cells in solid groups were the most commonly seen MyEC (44,6%). Those tumours found in the submandibular salivary glands contained 2.773 cells, of them, 993 were S-100 protein positive (35,8%) and plasmocytoid cells were the most commonly seen MyEC. Relative frequency of other cells in the studied tumours is also presented in table 4.
Discussion
There are many studies on the immunohistochemical features o the MyECs using numerous monoclonal an polyclonal antibodies as cytokeratins (CK-7,8,14, AE1-AE3, K18.12, KL1), muscle specific proteins as alpha-smooth muscle actin, muscle specific actin (HHF-35), calponin, myoglobin, smooth muscle myosin, smooth muscle myosin heavy chains, S-100 protein and its subunits, vimentin, P63, CD10, D2-40, laminin, maspin, MNF116, EMA, collagen type IV, H-caldesmon, iNOS, ß-catenin and others (9,15-27). All these studies showed that MyECs showed widely variable results (some times they were positive, and some times they were negative) and also, these studies showed they showed differences in the intensity of the cellular immunostaining. Results from these studies clearly demonstrate that MyECs have a very wide immunoprofile and that any of the used antibodies is specific to accurately detect the MyECs.
Writing on the histogenesis and electron microscopic features of the PA neoplastic cells, Dardick et al determined that putative MyECs found in chondroid and myxoid areas of their studied PA cases, these cells showed no satisfactory ultrastructural features of MyECs (10). In another study (11), Latkovich and Johnson found no positivity for markers to MyECs in a malignant PA. Hirano et al found that only few cells in their studied PAs showed positivity to actin antibodies (12). Palmer et al (13) demonstrated that MyECs were relatively rare in the majority of their studied PAs and that many of the cells, which have been classically described as MyECs in routine preparations, they did not clearly show this type of differentiation. In another study, Palmer et al (14) reported that ultrastructurally, typical MyECs were rarely encountered even in situations where they are reported to occur. In the Erlandson et al study (6), these authors reported that double immunostaining to vimentin and cytokeratin were seen in occasional MyECs. The above mentioned results and those figures from this study suggest that MyECs are not too common in the neoplastic tissues of the PAs as it is generally believed and that they are not the main neoplastic component of the PAs.
In our best knowledge, this is the first work quantitating the number of S-100 pro-tein positive MyECs in the neoplastic cell population of PAs. Our results demonstrate that MyECs only comprise approximately the 27,4% of the total neoplastic cell number of the studied PAs and that under the experimental conditions followed in this study, they do not constitute the main cellular component of the neoplastic compartment of the PAs.
Our cell quantitation showed that total number of cells counted in the analysed PAs is not related with age or gender of the patients. Tumours located in major salivary glands shower larger numbers of S-100 protein positive MyECs but, total numbers of positive cells in the analysed PAs are not related with location of the tumours. However, larger numbers of MyECs were found in 20 year-old or younger patients, a definitive correlation among the above mentioned parameters was not encountered.
Individual cell quantification of the MyECs found in the analysed PAs showed that plasmacytoid cells were the most abundant MyEC. As it is shown in this study, the rela-tive frequency of each type of MyEC varied in individual cases. Our results showed that the suspicion reported by other authors (10-14), regarding that MyECs are not the main cellular component of the neoplastic compartment in the PAs could be true. We propose it is necessary to achieve more studies quantifying PA neoplastic cells with different an-tibodies, in order to know the relative frequency of the different cells composing PAs.
References
1. Barnes L, Eveson J, Reichart P, Sidransky D eds. Pathology and Genetics. Head and Neck Tumours. Lyon: IARC Press; 2005. p. 210. [ Links ]
2. Rivera-Bastidas H, Ocanto RA, Acevedo AM. Intraoral minor salivary gland tumours. A retrospective study of 62 cases in a Venezuelan population. J Oral Pathol Med 1996;25:1-4.
3. Rippin JW, Potts AJC. Intra-oral salivary gland tumors in the West Midlands. Br Dent J 1992;173:17-9.
4. Eveson JW, Cawson RA. Tumors of the minor (oropharyngeal) salivary glands. A demographic study of 336 cases. J Oral Pathol 1985;14:500-9.
5. Ledesma-Montes C, Garcés-Ortiz M. Salivary gland tumors in a Mexican sample. A retrospective study. Med Oral 2002;7:324-30.
6. Erlandson RA, Cardon-Cardo C, Higgins PJ. Histogenesis of benign pleomorphic adenoma (mixed tumor) of the major salivary glands. Am J Surg Pathol 1984;8:803-20.
7. Kahn HJ, Baumal R, Marks A, Dardick I, van Nostrand AWP. Myoepithelial cells in salivary gland tumors. Arch Pathol Lab Med 1985;109:190-5.
8. Dardick I, van Nostrand AWP, Jeans D, Rippstein P, Edwards V. Pleomorphic adenoma. II. Ultrastructural organization of "stromal" regions. Human Pathol 1983;14:798-809.
9. Savera AT, Gown AM, Zarbo RJ. Immunolocalization of three novel smooth muscle-specific proteins in salivary gland pleomorphic adenoma: Assessment of the morphoge-netic role of myoepithelium. Mod Pathol 1997;10:1093-100.
10. Dardick I, van Nostrand AWP, Phillips MJ. Histogenesis of salivary gland pleomo-phic adenoma (mixed tumor) with an evaluation of the role of the myoepithelial cell. Hum Pathol 1982;13:62-75.
11. Latkovich P, Johnson RL. Carcinosarcoma of the parotid gland. Report of a case with cytologic and immunohistochemical findings. Arch Pathol Lab Med 1998;122:743-6.
12. Hirano T, Kashiwado I, Suzuki I, Yoshihiro T, Yuge K, Asano G. Immunohistopa-thological properties of pleomorphic adenoma in salivary gland. Nippon Ika Daigaku Zasshi 1990;57:172-9.
13. Palmer RM, Lucas RB, Knight J, Gusterson B. Immunocytochemical identification of cell types in pleomorphic adenoma, with particular reference to myoepithelial cells. J Pathol 1985; 146:213-20.
14. Palmer RM, Lucas RB, Langdon JD. Ultrastructural analysis of salivary gland pleomorphic adenoma, with particular reference to myoepithelial cells. Histopathology 1985;9:1061-76.
15. Furuse C, Cury PR, de Araujo NS, de Araujo VC. Application of two different clones of vimentin to the diagnosis of salivary gland tumors. Appl Immunohistochem Mol Morphol 2006;14:217-9.
16. de Araujo VC, Altemani A, Furuse C, Martins MT, de Araujo NS. Immunoprofile of reactive salivary myoepithelial cells in intraductal areas of carcinoma ex-pleomorphic adenoma. Oral Oncol 2006;42:1011-6.
17. Margaritescu C, Raica M, Simionescu C, Mogoanta L, Surpateanu M, Jaubert F, et al. Tumoral stroma of salivary pleomorphic adenoma - histopathological, histochemical and immunohistochemical study. Rom J Morphol Embryol 2005;46:211-23.
18. Furuse C, Sousa SO, Nunes FD, Magalhaes MH, Araujo VC. Myoepithelial cell markers in salivary gland neoplasms. Int J Surg Pathol 2005;13:57-65.
19. Ogawa Y, Kishino M, Atsumi Y, Kimoto M, Fukuda Y, Ishida T, et al. Plasmacytoid cells in salivary-gland pleomorphic adenomas: evidence of luminal cell differentiation. Virchows Arch 2003;443:625-34.
20. Huang J. Expression of S-100 proteins and intermediate filament proteins in pleomorphic adenoma. Zhonghua Kou Qiang Yi Xue Za Zhi 2000;35:191-3.
21. de Araujo VC, de Sousa SO, Carvalho YR, de Araujo NS. Application of immuno-histochemistry to the diagnosis of salivary gland tumors. Appl Immunohistochem Mol Morphol 2000;8:195-202.
22. Brennan PA, Umar T, Zaki GA, Langdon JD, Spedding A, Buckley J, et al. Are myoepithelial cells responsible for the widespread expression of inducible nitric oxide synthase in pleomorphic adenoma? An immunohistochemical study. J Oral Pathol Med 2000;29:279-83.
23. Takeda Y, Shimono M. Pleomorphic adenoma with nuclear palisading arrangement of modified myoepithelial cells: histopathologic and immunohistochemical study. Bull Tokyo Dent Coll 1999;40:27-34.
24. Nakayama H, Miyazaki E, Hiroi M, Kiyoku H, Naruse K, Enzan H. Socalled neoplastic myoepithelial cells in chondroid syringomas/mixed tumors of the skin: their sub-types and immunohistochemical analysis. Pathol Int 1998;48:245-53.
25. Huang JW, Ming Z, Shrestha P, Mori M, Ilg E, Schafer BW, et al. Immunohisto-chemical evaluation of the Ca(2+)-binding S-100 proteins S-100A1, S-100A2, S-100A4, S-100A6 and S-100B in salivary gland tumors. J Oral Pathol Med 1996;25:547-55.
26. Alos L, Cardesa A, Bombi JA, Mallofre C, Cuchi A, Traserra J. Myoepithelial tumors of salivary glands: a clinicopathologic, immunohistochemical, ultrastructural, and flow-cytometric study. Semin Diagn Pathol 1996;13:138-47.
27. do Prado RF, Consolaro A, Taveira LAA. Expression of ß-catenin in carcinoma in pleomorphic adenoma, pleomorphic adenoma and normal salivary gland: An immuno-histochemical study. Med Oral Patol Oral Cir Bucal 2006;11:E247-51.
Acknowledgements.
The authors are indebted with the personnel of the Laboratories of Experimental Pathology and the Laboratory of Neuroscience (Hospital de Oncología and Hospital de Especialidades, Centro Médico Nacional Siglo XXI) and those of the Laboratory of Clinical and Experimental Pathology (Facultad de Odontología, UNAM) for their technical assistance during the preparation of the slides.
![]() Correspondence:
Correspondence:
Dr. Constantino Ledesma-Montes.
Ciprés #169-2
Col. Vergel Coapa.
México, D.F. 14320.
MÉXICO.
E-mail: cotita@avantel.net
Received: 30-05-2006
Accepted: 15-11-2006