Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Medicina Oral, Patología Oral y Cirugía Bucal (Internet)
versión On-line ISSN 1698-6946
Med. oral patol. oral cir.bucal (Internet) vol.12 no.3 may. 2007
Antibiotic use in dental practice. A review
Rafael Poveda Roda1, José Vicente Bagán2, José María Sanchis Bielsa1, Enrique Carbonell Pastor1
(1) Physician and dentist. Service of Stomatology, Valencia University General Hospital
(2) Chairman of Oral Medicine, Valencia University, and Head of the Service of Stomatology, Valencia University General Hospital. Valencia
ABSTRACT
Antibiotics are commonly used in dental practice. It has been estimated that 10% of all antibiotic prescriptions are related with dental infections. The association amoxicillin-clavulanate was the drug most frequently prescribed by dentists during 2005, at least in the Valencian Community (Spain). The use of antibiotics in dental practice is characterized by empirical prescription based on clinical and bacteriological epidemiological factors, with the use of broad spectrum antibiotics for short periods of time, and the application of a very narrow range of antibiotics. The simultaneous prescription of nonsteroidal antiinflammatory drugs (NSAIDs) can modify the bioavailability of the antibiotic. In turn, an increased number of bacterial strains resistant to conventional antibiotics are found in the oral cavity.
Antibiotics are indicated for the treatment of odontogenic infections, oral non-odontogenic infections, as prophylaxis against focal infection, and as prophylaxis against local infection and spread to neighboring tissues and organs.
Pregnancy, kidney failure and liver failure are situations requiring special caution on the part of the clinician when indicating antibiotic treatment.
The present study attempts to contribute to rational antibiotic use, with a review of the general characteristics of these drugs.
Key words: Antibiotic, infection, odontogenic, prophylaxis.
RESUMEN
Los antibióticos son fármacos de uso cotidiano en odontología. Se estima que el 10% de las prescripciones antibióticas están relacionadas con la infección odontogénica. La asociación amoxicilina-clavulánico fue el fármaco más prescrito por dentistas durante 2005, al menos en la Comunidad Autónoma Valenciana. El uso de antibióticos en odontología se caracteriza por una prescripción empírica basada en epidemiología clínica y bacteriana, el uso de antibióticos de amplio espectro durante periodos breves de tiempo y el manejo de una batería muy reducida de antibióticos. La prescripción simultánea de AINES (antiinflamatorios no esteroideos) puede modificar la biodisponibilidad del antibiótico. Se detecta un aumento de número de cepas resistentes a los antibióticos convencionales en la cavidad oral.
La indicación antibiótica se realiza para tratamiento de la infección odontogénica, de infecciones orales no odontogénicas, como profilaxis de la infección focal y como profilaxis de la infección local y la extensión a tejidos y órganos vecinos.
El embarazo, la insuficiencia renal y la insuficiencia hepática son situaciones que requieren una especial atención del clínico antes de indicar un tratamiento antibiótico.
El objetivo del presente trabajo es intentar contribuir a un uso racional de los antibióticos revisando sus características generales.
Palabras clave: Antibiótico, infección, odontogénica, profilaxis.
Introduction
Antibiotic treatment is an aspect of pharmacotherapy with the particularity of affording both etiological and curative action. It was introduced in the mid-twentieth century in the form of sulfa drugs (1935), penicillin (1941), tetracyclines (1948) and erythromycin (1952). Since then, antibiotics have focused much clinical and pharmacological research, in response to the progressive challenges posed by bacterial infections: identification of new pathogens, the development of resistances to antibiotics, the consolidation of new diseases, and novel clinical situations (increase in chronic processes, survival of patients with disorders considered to be fatal until only recently, etc.) (1).
A good example of the usefulness of these drugs is provided by the fact that in the period 1998-2000, the number of daily doses of antibiotics per 1000 inhabitants was 30.7 with a cost of 47.18 euros/1000 inhabitants/day. Furthermore, in Spain during the year 2004, the public National Health Care System prescribed 25.61 million containers of macrolides, combinations of penicillins, other betalactams and fluorquinolones, with a total cost of 336.12 million euros (2). The fact that no antibiotic is included among the 35 most widely consumed generic drug products during the year 2004 is misleading. This is because antibiotics are generally prescribed for acute episodes and for brief periods of time, while the most heavily consumed medicines are those prescribed for chronic processes (antihypertensive agents, hypolipidemic drugs, antacids, antiinflammatory drugs, bisphosphonates, bronchodilators, etc.).
Bacterial infections are common in dental and oral clinical practice; as a result, antibiotic use prescribed for their treatment is also frequent. In Spain, it has been estimated that odontogenic infections are the cause of 10% of all antibiotic prescriptions (3).
In the Valencian Community (Spain), dentists in the public health care system during the year 2005 prescribed a total of 43,490 antibiotic containers, with a total cost of 274,439.82 euros. In relative terms, these figures represent 0.94% of the total antibiotic containers and 0.51% of the total antibiotic expenditure generated by the public health care system in the Valencian Community. By pharmaceutical specialties or drug products, amoxicillin and the association amoxicillin-clavulanic acid accounted for 67.8% of all prescriptions and 59.4% of the global cost. The association amoxicillin-clavulanic acid was the most frequently prescribed treatment, representing 38.7% of all prescriptions and 45.7% of the net cost. Spiramycin and the association spiramycin and metronidazole in turn accounted for 13.34% of the prescriptions and 10.2% of the global expenditure. Lastly, clindamycin represented 4% of the prescriptions and 4.2% of the costs. In sum, three drug substances and two drug associations or combinations of these same three drug substances account for 95% of all antibiotic prescriptions made by dentists in the context of the public health care system, and 75% of the total antibiotic cost.
The present study reviews antibiotic use in dental practice, and contributes elements to favor the rational use of such medicines.
Particularities of antibiotic use in dental and oral clinical practice
Dentist use of antibiotics is characterized by a number of particularities. In effect, antibiotic prescription is empirical, i.e., the clinician does not know what microorganism is responsible for the infection, since pus or exudate cultures are not commonly made. Based on clinical and bacterial epidemiological data, the germs responsible for the infectious process are suspected, and treatment is decided on a presumptive basis, fundamented on probabilistic reasoning (4).
As a result of the above, broad spectrum antibiotics are typically prescribed. A broad range of organisms can be isolated from the oral cavity, and although not all of them are potential human pathogens, the list of bacteria related with oral infections is relatively long (cocci, bacilli, grampositive and gramnegative organisms, aerobes and anaerobes).
As has been commented above, a very limited range of drug products is typically used – sometimes as few as two or three antibiotics. In turn, prescription is characteristically made for short periods of time – typically no more than 7-10 days.
The antibiotic sensitivity of the bacteria found within the oral cavity is gradually decreasing, and a growing number of resistant strains is detected – particularly Porphyromona and Prevotella (5), though the phenomenon has also been reported for Streptoccocus viridans and for drugs such as the macrolides, penicillin and clindamycin (6,7).
Antibiotic prescription is almost invariably associated with the prescription of nonsteroidal antiinflammatory drugs (NSAIDs). There are many potential interactions between these two drug categories – the most common situation being an NSAID-mediated reduction of antibiotic bioavailability and thus effect (8,9), though some combinations of drugs such as cephalosporins and ibuprofen, or tetracyclines with naproxen or diclofenac, have been shown to exert the opposite effect, i.e., an increase in the bioavailability of the antibiotic (10,11).
The target: microorganism
The human oral cavity contains a very broad range of germs. In effect, some authors speak of more than 500 different species, and Liebana even reports that all known microorganisms related to the human species are at some time isolated from the oral cavity as either transient (the majority) or resident species (only a few) (12). Despite this great variety of germs, those most commonly isolated from oral, dental, apical and periodontal exudates and pus are more limited in number – comprising organisms considered to be more pathogenic and which focus the majority of studies on antibiotic efficacy.
Isla et al. (13) compared the efficacy of antibiotics commonly used in dental and oral clinical practice in application to the bacteria most frequently isolated in odontogenic infections (S. viridans, Peptostreptococcus spp, Prevotella intermedia, Porphyromona gingivalis and Fusobacterium nucleatum), based on pharmacokinetic and pharmacodynamic (PK/PD) analyses (effect of the human body upon the drug, reflected by the plasma concentration profile -pharmacokinetics-, and the effect of the drug upon the body, as defined by the minimum inhibitory concentration, or MIC -pharmacodynamics-. On the basis of the results of their study, the authors suggested the recommended clindamycin dose to be 300 mg/6 hours, and 500 mg/8 hours or 2000 mg/12 hours for amoxicillin-clavulanic acid (with 125 mg of clavulanate in both cases). In turn, they reported that the association spiramycin-metronidazole at the usual dosage fails to cover the full bacterial spectrum in infections of this kind. The authors concluded that amoxicillin-clavulanic acid, clindamycin and moxifloxacin are the antibiotics of choice for the treatment of odontogenic infections – though they also pointed to the need for clinical trials to confirm the usefulness of PK/PD studies in these processes.
Bresco-Salinas et al. (5), in a clinical study of 64 patients with acute infection of pulp origin or pericoronaritis, found the germs most commonly isolated from the infection zone to be Streptoccocus, Enterococcus, Bacteroides, Fusobacterium, Porphyromonas, Prevotella and Actinobacillus. In the study of sensitivity to different antibiotics, they found amoxicillin and the association amoxicillin-clavulanic acid to offer very good results in the in vitro control of most of the germs identified (resistances < 10%) – though for Bacteroides and Prevotella intermedia the bacterial resistance rate was in the range of 25%. Antibiotics commonly used in dental practice, such as erythromycin, metronidazole or azithromycin, were found to be ineffective in application to over 30% of the strains (39.1%, 50.5% and 33.2%, respectively). Linezolid was the antibiotic with the best performance, proving effective in 94.6% of the strains. This antibiotic belongs to the family of oxazolidinones, which act by inhibiting protein synthesis, and which are effective against multiresistant grampositive germs and anaerobes. Linezolid is marketed in Spain under the brand name of Zyvoxid® (14). The authors consider amoxicillin to be the drug of choice in processes of this kind, and that clindamycin should be the alternative in the event of treatment failure or of patient allergy to penicillin.
Slightly divergent results have been published by Liñares and Martin-Herrero (15), who considered amoxicillin-clavulanate to be the option with the fewest resistant strains. Amoxicillin shows resistances in 30-80% of all strains of Prevotella and Porphyromona, and the macrolides are scantly effective. However, in this study, clindamycin and metronidazole were seen to be active against all the pathogens examined, except Actinobacillus actinomycetemcomitans.
More rotund findings have been reported by Sobottka et al. (16), who after isolating 87 pathogens from 37 patients with odontogenic abscesses, found 100% to be sensitive to amoxicillin-clavulanic acid. Excellent results were also obtained with fluorquinolones (moxifloxacin and levofloxacin), with sensitivity in 98% of all strains. The results were somewhat more discrete (sensitivity in the range of 70-75%) with doxycycline, clindamycin and penicillin.
Kirkwood, in a review on the use of antibiotics in orofacial infections (18), considered that although the penicillins traditionally have been used for the treatment of odontogenic infections, the growing presence of bacteria resistant to penicillin have caused other antibacterials – particularly clindamycin – to become the drugs of choice for treating infections of this kind, due to their good tolerance, low emergence of resistances, and the high drug concentrations reached in bone. In contrast to the above, Swift et al. (19) indicate that despite the recent introduction of many new antimicrobials, none have demonstrated significant benefit justifying their replacement of penicillin derivatives in application to orofacial infections. Furthermore, they consider that the appropriate use of these drugs, together with surgery, constitute adequate treatment for odontogenic infections.
To summarize, and as pointed out by Morcillo (19), a polymicrobial flora has been described in odontogenic infections, with strict anaerobes, and with a relatively limited microbial spectrum (despite the enormous variety of bacteria that transit through or colonize the oral cavity). This means that of the broad range of antibacterials available, a few drugs will suffice to treat odontogenic infections despite the empirical approach to management.
Table 1 reports the antibiotics most commonly used in dental practice, with an indication of the corresponding doses.
Indications of antibiotic treatment
The drawback to the evident benefits of antibiotic treatment is represented by the undesired effects of their use. On one hand there are side effects with repercussions for the patient, such as gastric, hematological, neurological, dermatological, allergic and other disorders. On the other hand, the development of bacterial resistances is of great importance for both individual patient and public health – the paradigm in this case being the ß-lactamase producing bacterial strains. As was demonstrated by Kuriyama et al. (20), ß-lactamase producing bacteria are isolated with increased frequency from the purulent exudate of odontogenic infections in patients that have received previous treatment with beta-lactams, and the longer the duration of such prior treatment the greater the number of resistant bacterial strains isolated.
Rational antibiotic use is thus required in dental and oral clinical practice, to ensure maximum efficacy while at the same time minimizing the side effects and the appearance of resistances.
Antibiotics are typically prescribed in dental practice for some of the following purposes: (a) as treatment for acute odontogenic infections; (b) as treatment for non-odontogenic infections; (c) as prophylaxis against focal infection in patients at risk (endocarditis and joint prostheses); and (d) as prophylaxis against local infection and systemic spread in oral surgery.
Treatment of the acute odontogenic infection
Despite the high incidence of odontogenic infections, there are no uniform criteria regarding the use of antibiotics to treat them. Bascones et al. (21), in a consensus document on the subject, suggested that treatment should be provided in some acute situations of odontogenic infection of pulp origin as a complement to root canal treatment, in ulcerative necrotizing gingivitis, in periapical abscesses, in aggressive periodontitis, and in severe infections of the fascial layers and deep tissues of the head and neck.
They do not recommend antibiotic treatment in chronic gingivitis or periodontal abscesses (except in the presence of dissemination).
There is considerable agreement that the beta-lactam derivatives are the antibiotics of choice for these processes, provided there are no allergies or intolerances. However, there is less consensus regarding which drug belonging this family should be prescribed. While some authors consider the natural and semisynthetic penicillins (amoxicillin) to be the options of first choice (22), others prefer the association amoxicillin-clavulanate, due to the growing number of bacterial resistance, as well as its broad spectrum, pharmacokinetic profile, tolerance and dosing characteristics (23). As has been commented above, some authors have proposed clindamycin as the drug of choice, in view of its good absorption, low incidence of bacterial resistances, and the high antibiotic concentrations reached in bone (17).
Treatment of non-odontogenic infections
Non-odontogenic infections include specific infections of the oral cavity (tuberculosis, syphilis, leprosy), and nonspecific infections of the mucosal membranes, muscles and fascias, salivary glands and bone. Bone infections are included here on the grounds that many of them may be of dental origin. These processes require prolonged treatments, and drug associations are used that usually include clindamycin, due to its capacity to reach high concentrations in bone (24), and fluorquinolones (ciprofloxacin, norfloxacin, moxifloxacin) – to extend the bacterial spectrum to include gramnegative bacilli, grampositive aerobic cocci and, in the case of third generation fluorquinolones (moxifloxacin), anaerobes (25).
An anecdotal observation is that Bystedt et al. (24) found the maximum mandibular concentration of antibiotic to correspond to doxycycline, with 2.6 µm/gram, versus 0.6 µm/gram of clindamycin.
It is recommended that empirical treatment with betalactams associated to fluorquinolones should be limited, since both groups of antibiotics activate common resistance mechanisms – thus favoring the appearance of resistances in important pathogens such as Pseudomona aeruginosa and Acinetobacter spp (26).
The treatment of specific infections caused by mycobacteria requires the use of antibiotics for long periods of time (from 6 months to 2 years), and includes the administration of dapsone (a sulfamide analog), clofazimine (a dye with bactericidal action) and rifampicin for leprosy, and associations of ethambutol, isoniazid, rifampicin, pyrazinamide and streptomycin for tuberculosis (27). The treatment of syphilis, caused by Treponema pallidum, is based on the use of penicillin G benzatine. Administration comprises 2.4 million IU in a single intramuscular dose in the primary period, three doses of 2.4 million IU via the intramuscular route, spaced one week apart, in the secondary period. In the tertiary period a first treatment is provided with intravenous penicillin G, followed by penicillin G benzatine via the intramuscular route once a week during 3 weeks, involving a dose of 2.4 million IU each.
Prophylaxis of focal infection
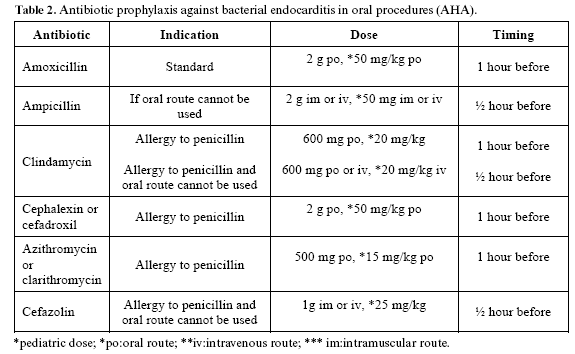
The use of antibiotics as prophylaxis for focal infection is common practice, and has been widely accepted in the dental profession. The paradigm of this model of treatment is the prevention of bacterial endocarditis, indicated in risk patients in the context of any invasive procedure within the oral cavity – and following the guidelines of the American Hearth Association (AHA) (28) (Table 2).
Nevertheless, there are doubts in relation to this practice. Firstly, transient bacteremia occurs not only after dental treatments such as extractions (35-80%) or periodontal surgery (30-88%). It also occurs in the context of tooth brushing (40%) or while chewing gum (20%), and is proportional to the trauma caused and to the number of germs colonizing the affected zone. Secondly, not only bacteria cause endocarditis, and of those that do cause the disease, many are resistant to the antibiotics administered as prophylaxis (fundamentally amoxicillin). Lastly, it is known that most cases of bacterial endocarditis are not related with invasive procedures, and that dental care is only responsible for a minimum percentage of cases of the disease.
Despite the mentioned inconveniences, antibiotic prophylaxis is still recommended in patients at risk (29). However, the results of a survey conducted by Tomas-Carmona et al. (30) on the knowledge and approach to the prevention of bacterial endocarditis among Spanish dentists showed that fewer than 30% of the professionals were aware of correct antibiotic indications and posology.
There is no scientific basis for recommending systematic antibiotic prophylaxis prior to invasive dental treatment in patients with total joint prostheses (31). Jacobson published a study on 2693 patients with total joint replacement (hip or knee). In 30 of the patients he detected infection of the prosthesis, and in only one case was a time relationship with prior dental treatment established. Furthermore, 54% of the germs isolated were Staphylococcus aureus and epidermidis (32).
According to the American Dental Association and the American Academy of Orthopedic Surgeons, evaluation is required of antibiotic prophylaxis in patients with total joint prostheses in the presence of immune deficiency, when contemplating high risk dental procedures in patients with prostheses in place for less than two years, and in patients who have already suffered past joint prosthesis infections (33).
Prophylaxis of local infection and systemic spread
Prophylaxis of local infection is taken to comprise the administration of antibiotics on a pre-, intra- or postoperative basis, to prevent bacterial proliferation and dissemination within and from the surgical wound. Few clinical studies to date have evaluated this type of treatment. Some authors have reported its efficacy, with statistically significant differences in the frequency of infectious complications in surgical extractions of lower third molars between patients who had received some form of antibiotic treatment and those without (34).
In a retrospective study of infections following periodontal surgery in 390 patients and involving 1053 surgical procedures carried out by Powell et al. (35), the reported total frequency of infections was found to be 2.09% - no differences being recorded between those patients administered antibiotics perioperatively and those without. The authors therefore did not consider it to be justified to administer antibiotics on a postoperative basis with the sole purpose of avoiding postoperative infections in operations of this type, which included curettage with flap raising, the placement of implants, sinus lifting, soft tissue autografts and coronal displacement flaps.
In a consensus document on the use of antibiotic prophylaxis in dental surgery and procedures published in 2006 (36), prophylaxis in oral surgery in a healthy patient was only recommended in the case of the removal of impacted teeth, periapical surgery, bone surgery, implant surgery, bone grafting and surgery for benign tumors. In subjects with risk factors for local or systemic infection - including oncological patients, immune suppressed individuals, patients with metabolic disorders such as diabetes, and splenectomized patients, prophylactic antibiotic coverage should be provided before attempting any invasive procedure.
The use of antibiotics in endodontics should be reserved for patients with signs of local infection, malaise of fever. Prophylactic or preventive use should be reserved for endocarditis and the systemic disorders commented above – avoiding indiscriminate antibiotic use (37).
Precautions with antibiotic use
- Pregnancy
The legal and ethical impossibility of conducting clinical trials in humans to evaluate the risks of antibiotic treatment during pregnancy has given rise to uncertainties as to the use of such drugs in these patients. The United States Food and Drug Administration (FDA) has established four levels of drug risk during pregnancy: (A) without demonstrated risk; (B) without effects in animals, though with undemonstrated innocuousness in humans; (C) no studies conducted in either animals or humans, or teratogenic effects recorded in animals without due evaluation in humans; and (D) teratogenic effects upon the fetus – use of the drug being conditioned to the obtainment of benefit that outweighs the risks. A final group (X) in turn contemplates teratogenic effects that outweigh any possible benefit derived from the drug.
No antibiotic corresponds to group A. On the other hand, group B (i.e., warranting caution with treatment during pregnancy) contains the following antibiotics: azithromycin, cephalosporins, erythromycin, metronidazole and penicillins with or without beta-lactamase inhibitors. Group C in turn includes clarithromycin, the fluorquinolones and the sulfa drugs (including dapsone). Finally, group D contains the aminoglycosides and tetracyclines (38).
- Kidney failure
Many antibiotics are actively eliminated through the kidneys. The presence of impaired renal function requires reduction of the drug dose in order to avoid excessively elevated plasma drug concentrations that could lead to toxicity. dose adjustment can be carried out by reducing the amount administered in each dose or by increasing the interval between doses (without modifying the amount of drug). Neither approach has been shown to be superior (39).
Table 3 reports some of the antibiotics most frequently used in dental practice, with the dose adjustments required according to the degree of kidney failure (assessed according to creatinine clearance).
- Liver failure
Some antibiotics are metabolized in the liver, followed by elimination in bile. In patients with liver failure, the use of such antibiotics should be restricted in order to avoid toxicity secondary to overdose. Erythromycin, clindamycin, metronidazole and anti-tuberculosis drugs are antibiotics requiring dose adjustments when administered to patients with liver failure.
Regardless of the above considerations, some antibiotics are potentially hepatotoxic. As a result, and whenever possible, they should be avoided in patients with some active liver disorder. Specifically, tetracyclines and anti-tuberculosis drugs should be avoided (40).
References
1. Morcillo E, Cortijo J, Villagrasa V. Bases farmacológicas de la antibioticoterapia en infecciones odontogénicas. Med Oral 1996;1:15-23. [ Links ]
2. Grupos terapéuticos de mayor consumo en el Sistema nacional de Salud durante 2004. Información Terapéutica del Sistema nacional de Salud 2005;29:49-53. [ Links ]
3. Machuca M, Espejo, Gutierrez L, Herrera J. Análisis de la prescripción antibiótica en una farmacia comunitaria. Pharm Care Esp 2000;18:300-7. [ Links ]
4. Vallano A, Izarra A. Principios de terapéutica antimicrobiana. Medicine 2006;9:3196-203. [ Links ]
5. Bresco-Salinas M, Costa-Riu N, Berini-Aytes L, Gay-Escoda C. Susceptibilidad antibiótica de las bacterias causantes de infecciones odontogénicas. Med Oral Patol Oral Cir Bucal 2006;11:51-6. [ Links ]
6. Aracil B, Minambres M, Oteo J, Torres C, Gomez-Garces JL, Alos JI. High prevalence of erythromycin-resistant and clindamycin-susceptible (M phenotype) viridans group streptococci from pharyngeal samples: a reservoir of mef genes in commensal bacteria. J Antimicrob Chemother 2001;48:592-4. [ Links ]
7. Groppo FC, Castro FM, Pacheco AB, Motta RH, Filho TR, Ramacciato JC, et al. Antimicrobial resistance of Staphylococcus aureus and oral streptococci strains from high-risk ndocarditis patients. Gen Dent 2005;53:410-3. [ Links ]
8. Groppo FC, Simoes RP, Ramacciato JC, Rehder V, de Andrade ED, Mattos-Filho TR. Effect of sodium diclofenac on serum and tissue concentration of amoxicillin and on staphylococcal infection. Biol Pharm Bull 2004;27:52-5. [ Links ]
9. de Cassia Bergamaschi C, Motta RH, Franco GC, Cogo K, Montan MF, Ambrosano GM, et al. Effect of sodium diclofenac on the bioavailability of amoxicillin. Int J Antimicrob Agents 2006;27:417-22. [ Links ]
10. Tsivou E, Melakopoulos I, Kotsiou A, Anagnostopoulou S, Tesseromatis C. Alterations in cefalosporin levels in the serum and mandible of hyperlipaedemic rats after co-administration of ibuprofen. Eur J Drug Metab Pharmacokinet 2005;30:171-4. [ Links ]
11. Oh YH, Han HK. Pharmacokinetic interaction of tetracycline with non-steroidal anti-inflammatory drugs via organic anion transporters in rats. Pharmacol Res 2006;53:75-9. [ Links ]
12. Liébana-Ureña J, González MP, Liébana MJ, Parra LE. Composición y ecología de la microbiota oral. En: Liébana-Ureña J Ed. Microbiología Oral. Madrid:McGraw-Hill; 2002. p. 514-25. [ Links ]
13. Isla A, Canut A, Rodriguez Gascon A, Pedraz JL. Análisis farmacocinético/framacodinámico (PK/PD) de la antibioterapia en odontoestomatología. Enferm Infecc Microbiol Clin 2005;23:116-21. [ Links ]
14. Catálogo de Medicamentos. Colección Consejo Plus Tomo I. Madrid: Consejo General de Colegios Oficiales de Farmacéuticos;2006. p.1594-6 [ Links ]
15. Liñares R, Martín-Herrero JE. Bases farmacológicas del tratamiento antibiótico de las enfermedades periodontales y periimplantarias. Av Odontoestomatol 2003; especial:23-33. [ Links ]
16. Sobottka I, Cachovan G, Sturenburg E, Ahlers MO, Laufs R, Platzer U, et al. In vitro activity of moxifloxacin against bacteria isolated from odontogenic abscesses. Antimicrob Agents Chemother 2002;46:4019-21. [ Links ]
17. Kirkwood KL. Update on antibiotics used to treat orofacial infections. Alpha Omegan 2003;96:28-34. [ Links ]
18. Swift JQ, Gulden WS. Antibiotic therapy--managing odontogenic infections. Dent Clin North Am 2002;46:623-33. [ Links ]
19. Morcillo E. Fundamentos farmacológicos de la terapéutica antimicrobiana. Av Odontoestomatol 1997;Sppl A:29-35. [ Links ]
20. Kuriyama T, Nakagawa K, Karasawa T, Saiki Y, Yamamoto E, Nakamura S. Past administration of beta-lactam antibiotics and increase in the emergence of beta-lactamase-producing bacteria in patients with orofacial odontogenic infections. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2000;89:186-92. [ Links ]
21. Bascones Martinez A, Aguirre Urizar JM, Bermejo Fenoll A, Blanco Carrion A, Gay-Escoda C, Gonzalez-Moles MA, et al. Documento de consenso sobre el tratamiento antimicrobiano de las infecciones bacterianas odontogénicas.Med Oral Patol Oral Cir Bucal 2004;9:369-76. [ Links ]
22. Berini L, Gay C. Normas generales de tratamiento de la infección odontogénica. Antibioticoterapia. Profilaxis de las infecciones postquirúrgicas y a distancia. En:Gay C, Berini L, eds. Tratado de Cirugía Bucal. Tomo I. Madrid:Ergón; 2004. p. 617-38. [ Links ]
23. Maestre-Vera JR. Opciones terapéuticas en la infección de origen odontogénico. Med Oral Patol Oral Cir Bucal 2004;9 Suppl:19-31. [ Links ]
24. Bystedt H, DAhlback A, Dornbusch K, Nord CE. Concentrations of azidocillin, erythromycin, doxycycline and clindamycin in human mandibular bone. Int J Oral Surg 1978;7:442-9. [ Links ]
25. Parra J, Peña A, Martínez MA, Hernández J. Quinolonas, sulfamidas, trmetoprima, cotrimoxazol. Medicine 2006;9:3538-43. [ Links ]
26. Peterson LR. Squeezing the antibiotic balloon: the impact of antimicrobial classes on emerging resistance. Clin Microbiol Infect 2005;11:4-16. [ Links ]
27. Rozman C. Compendio de Medicina Interna. Madrid: Elsevier 2006. p. 683-90. [ Links ]
28. Dajani AS, Taubert KA, Wilson W, Bolger AF, Bayer A, Ferrieri P, et al .Prevention of bacterial endocarditis: recommendations by the American Heart Association. J Am Dent Assoc 1997;128:1142-51. [ Links ]
29. ADA. Terapéutica Dental. Barcelona:Masson; 2003. p. 596-600 [ Links ]
30. Tomas Carmona I, Diz Dios P, Limeres Posse J, Outumuro Rial M, Caamano Duran F, Fernandez Feijoo J, et al. Chemoprophylaxis of bacterial endocarditis recommended by general dental practitioners in Spain. Med Oral 2004;9:56-62. [ Links ]
31. Pallasch TJ, Slots J. Antibiotic prophylaxis and the medically compromised patient. Periodontol 2000 1996;10:107-38. [ Links ]
32. Jacobson JJ, Millard HD, Plezia R, Blankenship JR. Dental treatment and late prosthetic joint infections. Oral Surg Oral Med Oral Pathol 1986;61:413-7. [ Links ]
33. American Dental Association; American Academy of Orthopedic Surgeons. Antibiotic prophylaxis for dental patients with total joint replacements. J Am Dent Assoc 2003;134:895-9. [ Links ]
34. Martínez Lacasa J, Jiménez, Ferrás VA, Garcia Rey G, Bosom M, Solá-Morales O et al. A double-blind placebo-controlled, randosmised comparative phase III clinical trial of pharmacokinetiocally enhanced amoxicillin-clavulanate 2000/125, as prophilaxis or as treatment versus placebo for infectious and inflammatory morbidity after third molar mandibular removal. Program and abstracs of the 43rd InterScience Conference on Antimicrobial Agents and Chemotherapy. Chicago 2003. American Society for Mocrobiology, Washington DC. Citado en: Gutiérrez JL, Bagán JV, Bascones A, Llamas R, Llena J, Morales A, et al. Documento de consenso sobre la utilización de profilaxis antibiótica en cirugía. Med Oral Patol Oral Cir Bucal 2006;11:119-36. [ Links ]
35. Powell CA, Mealey BL, Deas DE, McDonnell HT, Moritz AJ. Post-surgical infections: prevalence associated with various periodontal surgical procedures. J Periodontol 2005;76:329-33. [ Links ]
36. Gutierrez JL, Bagan JV, Bascones A, Llamas R, Llena J, Morales A et al. documento de consenso sobre la utilización de profilaxis antibiótica en cirugía y procedimientos dentales. Med Oral Patol Oral Cir Bucal 2006;11:119-36. [ Links ]
37. Abbott PV, Hume WR, Pearman JW. Antibiotics and endodontics. Aust Dent J 1990;35:50-60. [ Links ]
38. Michavila A, Flórez J, García-Lobo JM. Farmacología de las enfermedades infecciosas. Principios generales, selección y asociación de antibióticos. En: Flórez J. Ed. Farmacología humana 4ªed. Barcelona: Masson SA; 2005. p. 1081-103. [ Links ]
39. Livornese LL Jr, Slavin D, Gilbert B, Robbins P, Santoro J. Use of antibacterial agents in renal failure. Infect Dis Clin North Am 2004;18:551-79. [ Links ]
40. Douglas LR, Douglas JB, Sieck JO, Smith PJ. Oral management of the patient with end-stage liver disease and the liver transplant patient. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1998;86:55-64. [ Links ]
![]() Correspondence:
Correspondence:
Dr. Rafael Poveda Roda
Servicio de Estomatología.
Hospital General Universitario de Valencia.
Av/ Tres Cruces nº 4
46014 Valencia
E-mail: poveda_raf@gva.es
Received: 8-08-2006
Accepted: 9-12-2006