Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Medicina Oral, Patología Oral y Cirugía Bucal (Internet)
versión On-line ISSN 1698-6946
Med. oral patol. oral cir.bucal (Internet) vol.12 no.3 may. 2007
Oral aphthous-like ulceration due to tiotropium bromide
Vanja Vucicevic Boras, Neil Savage, Zuraiza Mohamad Zaini
Department of Oral medicine, School of Dental medicine, 200 Turbot Street, University of Queensland, Brisbane, Australia
ABSTRACT
Unwanted side-effects of a drug therapy are well known to oral medicine specialists and other colleagues. Usually they manifest itself as dry mouth, taste disturbances, various allergic or toxic reactions on the lips and/or in the oral cavity. However, the list of the drugs which might induce unwanted reactions is everyday becoming longer as more and more drugs are introduced on the market. Certain problems when diagnosing and reporting unwanted side effects of the drugs exist as only accurate method of diagnosis is repeated drug use in controlled clinical setting where fatal consequences due to the anaphilactic shock could be avoided. We report a side effect reaction to tiotropium bromide (Spiriva®) cap used with HandiHaler manifesting itself as an oral ulceration in a 65 yrs old male. On the third day of drug intake the patient developed oral ulceration two times in a period of few months. Other medications he has been using for several years. To our knowledge this is a first report as an oral side-effect of this drug used for treatment of chronic obstructive pulmonary disease (COPD).
Key words: Oral ulceration, side effect of drug, tiotropium bromide.
Introduction
Virtually every drug has the potential to cause adverse reactions on the oral mucosa but some have greater ability to do so. So far, a wide range of drugs have been recognized as potential inducers of unwanted adverse reactions in the oral cavity. Smith and Burtner (1) reviewed such reactions after administration of 200 most frequently prescribed drugs and reported that the most freuquent ones were dry mouth (80.5%), dysgeusia (47.5%) and stomatitis (33.9%). However, other reactions to numerous drugs such as swellings, hypersalivation, discoloration of saliva, white lesions, oral burns, fixed drugs eruptions, mucositis, neoplasms, pemphigus and pemphigoid reactions and other bullous disorders, mucosal pigmentation, lichenoid reactions, cheilitis, neuropathies, and halitosis have been reported throughout the literature (2).
Drug-related aphthous-like ulceration have been reported after the use of beta blockers such as labetalol, captopril, nicorandil and non-steroidal anti-inflammatory drugs (NSAID). Also such reactions have been described after the use of mycophenolate or sirolimus, sodium lauryl sulfate, protease inhibitors, tacrolimus and sulfonamides, though the exact pathogenic mechanisms are unclear in all of these. A case control study has now confirmed the association of oral ulceration with NSAIDs and beta blockers, whereas all the other data are obtained from case reports, small series and non-peer-reviewed reports (2). Recently, we have reported a case report of a delayed contact sensitivity on the lips and oral mucosa due to propolis (3).
Case report
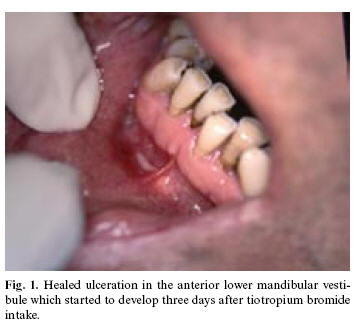
The patient, 65 years old was reviewed on the annual appointment for long-standing oral lichen planus at the Department of Oral medicine, University of Queensland. At the time his lesions of oral lichen planus were barely visible and only discrete whitish lines were seen on the lateral left side of the tongue. However, during the examination, he reported development of the ulceration in the vestibular mucosa in the region 41 and 42 which started after he took the third cap (by use of HandiHaler) of tiotropium bromide for his COPD symptoms. At the closer inspection the ulceration has now been in healing phase (Figure 1). Additionally patient reported that last time he was using tiotropium bromide also after 3 days of therapy, the ulceration developed. Given the clear relationship between appearence of the lesion and the administration of tiotropium bromide the patient was advised to stop taking it. The lesion resolved after ten days. Detailed medical history revealed that he suffered heart attack before 18 years and he had blood transfusion eleven years ago. From time to time he has lower back pain. Apart from that he has been using the same medications throughout many years such as diltiazem hydrochloride (Cardizem®; 240 mg/day), isosorbide dinitrate (Isordil®; 5mg/6x a day), candesartan cilexetil (Atacand®; 8mg/day), perindopril erbumine (Coversyl® plus; 4/1.25 a day), aspirin (Astrix®; 100 mg/day), biperiden hydrochloride (Akineton®; 2mg/day). To date, 3 months from our patients last review no such lesions developed and patient stopped taking tiotropium bromide. A biopsy was not taken because there was a clear relationship between the drug use and oral lesion.
Discussion
Long-acting bronchodilators are the mainstream for the treatment of COPD nowadays, and in the last 3 years tiotropium bromide has been put on the market in Western countries (1). So far, it has been documented that tiotropium bromide results in dry mouth in approximatelly 10-16% of the patients which is reversible and rarely causes discontinuation of therapy (2). Additionally drug may have various side effects involving skin (rash, urticaria and pruritus), urinary difficulties and retention, constipation, blurred vision and glaucoma, increased heart rate and cough as well as throat iritation. Also hypersensitivity reactions including isolated cases of angioedema have been reported (4). Most recently, subacute cutaneous lupus erythematosus through inhalation route has been described in one patient and paralytic ileus in another (5,6). Despite enhanced specific chemical effects of muscarinic drugs, side-effects on other organs can not be avoided completely as confirmed in this case report.
References
1. Smith RG, Burtner AP. Oral side-effects of the most frequently prescribed drugs. Spec Care Dent 1994;14:96-102. [ Links ]
2. Scully C, Bagan JV. Adverse drug reactions in the orofacial region. Crit Rev Oral Biol Med 2004;15:221-39. [ Links ]
3. Brailo V, Vucicevic-Boras V, Alajbeg I, Vidovic-Juras D. Delayed contact sensitivity on the lips and oral mucosa due to propolis-case report. Med Oral Patol Oral Cir Bucal 2006;11:303-4. [ Links ]
4. Tashkin DP, Cooper CB. The role of long-acting bronchodilators in the management of stable COPD. Chest 2004;125:249-59. [ Links ]
5. Gross NJ. Tiotropium bromide. Chest 2004;126:1946-53. [ Links ]
6. Tashkin DP. Is a long-acting inhaled bronchodilator the first agent to use in stable chronic obstructive pulmonary disease. Curr Opin Pulm Med 2005;11:121-8. [ Links ]
![]() Correspondence:
Correspondence:
Dr.Vanja Vucicevic Boras
Dept. of Oral medicine
School of Dentistry
University of Queensland
200 Turbot Street
Brisbane, 4000, QLD
Australia
E-mail: borasvanja@yahoo.com
Received: 14-03-2006
Accepted: 30-01-2007