Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Medicina Oral, Patología Oral y Cirugía Bucal (Internet)
versión On-line ISSN 1698-6946
Med. oral patol. oral cir.bucal (Internet) vol.12 no.7 nov. 2007
The influence of soft acidic drinks in exposing dentinal tubules after non-surgical periodontal treatment: A SEM investigation on the protective effects of oxalate-containing phytocomplex
Salvatore Sauro1, Francesco Mannocci1, Timothy F. Watson1, Matteo Piemontese2, Martyn Sherriff1, Romano Mongiorgi3
(1) Kings College London Dental Institute at Guys, Kings College and St Thomass Hospitals, Department of Biomaterials and Biomimetic materials, London, UK
(2) Dental School, University Politecnica delle Marche, Ancona, Italy
(3) Department of Biomineralogy, Crystallography and Biomaterials, University of Bologna, Italy
ABSTRACT
Objective: The aim of this study was to investigate the different smear layer morphologies produced by instrumentation with a hand curette and a periodontal sonic scaler for potential removal by soft acidic solution. The effect of a new oxalate-containing phytocomplex spray in preventing tubules exposure after citric acid solution application was also evaluated.
Methods: Thirty recently extracted human teeth were used to obtain root dentinal fragments and divided in two groups: Curette treatment (CRT) root planed applying 30 working strokes to each surface using a Graceys curette 5-6 and Ultrasonic scaler (USC) treated using a periodontal scaler mounted on an ultrasonic hand-piece for 30 seconds. Each principal group was further divided in three sub-groups (Control, Acid challenge and Acid/Phyto-oxalate). The control group samples were immersed in distilled water buffered to pH 7.4 using NH4OH solution. The samples of the acid challenge group were immersed in a solution of citric acid 0,02M; [pH 2.5] for 3 minutes. The samples of the Acid/Phyto-oxalate group were sprayed for 15 sec with a 1.5% phytocomplex spray prior to immersion. Samples were examined using SEM.
Results: Ultrasonic instrumentation created a very thin smear layer whereas curettes produced a multilayered smear layer. The acidic solution was able to remove the smear layer from root surfaces treated with ultrasonic instrumentation exposing the dentinal tubules. The smear layer on the root surfaces treated with hand instruments was not completely removed. The phytocomplex solution was able to prevent dentinal tubule exposure.
Conclusions: Acidic soft drinks are able to remove the smear layer created on root surfaces during different non-surgical periodontally treatments. The smear plugs created by hand instrumentation appeared to be more resistant to acid attack. The tested phytocomplex solution protected the dentine from demineralization and it might prevent post-treatment dentinal hypersensitivity induced by acidic soft drinks.
Key words: Root, dentine hypersensitivity, acidic drinks, smear layer, curettes, ultrasonic instruments, non-surgical periodontal treatments, Phytocomplexes, Oxalates.
Introduction
Sonic and manual scaling and root planning are the main procedures in non-surgical periodontal therapy. These procedures aim to remove the plaque and calculus from the altered cementum of the root surfaces for the consequent healing events (1-14). The cement in the cervical region is very thin, ranging from 20 to 50 µm, even when intact and histologically normal. This layer offers very little protection against thermal shock or any other irritant and besides can be easily removed by periodontal curette, sonic scalars and even toothbrushing with abrasive paste (15). Removal of the exposed cementum is necessary to allow fibroblasts adhesion to previously diseased and non-diseased areas of the roots (3). Moreover, complete removal of the cementum from the root surface seems to be essential to re-establish periodontal health (14).
Although the periodontal healing process may result in gingival recession and subsequent root exposure, it is well known that the scaling and root planning procedures produce a layer of debris, the so-called "smear layer" that covers the surface of instrumented dentine and occlude the dentinal tubules. Dentinal tubule exposure is directly related to the dissolution of the smear layer which induces root dentine hypersensitivity (16-18). A study conducted by Prati et al. (19) has showed that acidic soft drinks may be responsible for dentine smear layer dissolution and increase dentine permeability with consequent risk of dentine hypersensitivity. Dentine hypersensitivity has been defined as a short sharp pain arising from exposed dentine in response to thermal, evaporative, tactile, osmotic or chemical stimuli and which may not be ascribed to any other form or dental defect or pathology (20, 21). Any substance that decreases dentinal conductance (i.e. dentinal permeability) by closing patent tubules is able to reduce dentine hypersensitivity (22, 23). Previous studies concerning oxalate-based treatments on exposed dentinal tissue showed significant reductions in dentinal permeability and hypersensitivity (24-32). It is well known that many vegetables, such as rhubarb, spinach and mint, contain oxalates either as soluble or insoluble salts or as oxalic acid (29, 30). Oxalic acid forms soluble salts with sodium, potassium or ammonium ions, and insoluble salts with calcium, magnesium and iron ions (31, 32). In neutral and alkaline environments, calcium and oxalate may bind together forming crystals of calcium oxalate.
No information is currently available on the effect of Phytocomplex oxalates-containing substances to prevent smear layer dissolution and consequent dentinal hypersensitivity after non surgical periodontal treatments.
This study aimed to investigate using scanning electron microscopy, the ultramorphology of the smear layer created on root surface by conventional periodontal curettes and periodontal sonic scalers. Furthermore, the effect of citric acid solution on the root-planed surface and on the surface treated with ultrasonic instruments with or without application of oxalate-containing phytocomplex spray was evaluated.
The null hypothesis to be tested is that an acidic soft drink was able to dissolve the smear layer created by non-surgical periodontal treatment with conventional periodontal curettes and sonic instruments. The second hypothesis tested was that application of phytocomplex oxalate-containing spray was able to prevent smear layer dissolution and dentinal tubules exposure induced by an acidic soft drink.
Materials and methods
Sample preparation
Thirty single-rooted teeth, recently extracted for orthodontic and surgical reasons were used in this study. The crowns were cut approximately 1 mm below the cementum enamel junction (CEJ) using a diamond saw under copious water-cooling. A second cut was used to remove the apex of the root in order to obtain samples with a surface area of approx. 5 mm2. Subsequently, a longitudinal cut was made in order to obtain 2 fragments from each root sample. The cementum from the cervical portion was removed using a high-speed diamond-coated bur in order to expose the dentine (Figure 1). The root-samples fragments were divided randomly into 2 principal groups of thirty fragments based on the type of non-surgical periodontal instrumentation (USC: ultrasonic scaler treatment; CRT: curette treatment). Each principal group was further divided into three sub-groups of ten samples each (Control, Acid challenge and Acid/phyto-oxalate). The samples were stored before the test for no longer than five days in distilled water buffered to pH 7.4 using 28% NH4OH solution (Carlo Erba, Milan – Italy).
The root planing procedure was completed by two different operators. The same operators created a smear layer by applying 30 working strokes to each surface of the first principal group (CRT) using a novel sharpened Graceys curette 5-6. The second group of samples (USC) was treated using a coated titanium nitride periodontal tip mounted on an ultrasonic hand-piece Piezolight 5 (Castellini, Castel Maggiore, Bologna, Italy) working at 25 e 32 kHz for 30 seconds (about 30 strokes) in a vertical direction. After each respective root planing treatment, the control subgroup samples were immersed in distilled water buffered to pH 7.4 using NH4OH solution. The samples of the experimental acid challenge subgroup were immersed in a solution of citric acid 0,02M; [pH 2.5] for 3 minutes. The samples of the experimental acid/phyto-oxalate subgroup were first sprayed for 15s with a phytocomplexs oxalate-containing solution (1.5% spinach and rhubarb); immersed in a solution of citric acid 0,02M [pH 2.5] for 3 minutes; washed with deionised water for 30 sec. The acidic solution was not buffered, since pH may be a factor in smear layer removal and, consequently, may influence dentinal sensitivity. The phytocomplex oxalate-containing solution was prepared using cry-lyophilised extracts of spinach leaves (Spinacia oleracia) and rhubarb stalks (Rhubarb rhaponicum) in powdered form. The experimental solution was made and provided by ABOCA Company (Sansepolcro, Arezzo, Italy).
Finally, all the samples were dehydrated in ascending alcohols, coated with gold, and directly examined using an SEM (Joel 5200, JEOL, Tokyo, Japan) at 5-10 kV. Each dentine fragment was considered as sample unit and 3 photomicrographs were obtained from the centre of each sample, with two different magnifications (X2000, X5000); these images were intended to be representative of the most common features observed in each sample. These photomicrographs were subsequently assessed by one examiner who was blind to the experimental procedure, using the following index of smear layer removal.
Grade 1: Complete removal of smear layer, dentinal tubules open.
Grade 2: Complete removal of smear layer, rare presence of smear plugs into dentinal tubules.
Grade 3: Partial removal of smear layer, dentinal tubules occluded
Grade 4: Smear layer present on the dentine surface, total obliteration of dentinal tubules.
The distribution of smear layer removal grades was tested using Pearsons c2 test and significance was established at p = 0.001 and adjusted residuals were used to identify significant treatment grade interactions. On the other hand, in order to evaluate the protective effect of the phytocomplex treatment, the number of exposed dentinal tubules was counted in five random pictures in each group. Each picture had a surface of 900 µm2 at a magnification of X2000. The effect of phytocomplex treatment on the number of exposed tubules was evaluated using the Kruskal-Wallis test with a multiple pair wise comparison of treatments with significance pre-determined at a= 0.05. The null hypothesis was that treatment has no effect on the number of exposed tubules.
Results
The scanning electron micrographic appearance of the root surface treated by curette instrumentation revealed a multilayered smear layer which obscured the orifices of dentinal tubules (Fig. 2A). Higher magnification images showed the presence of many grooves that gave the smear layer morphology similar to that of "tree-bark" (Fig. 2B). The root surface treated by ultrasonic instrumentation appeared characterised by many grooves and a thin layer of debris which only partially covered the root dentine (fig. 2C). Higher magnification images showed that the smear layer produced by ultrasonic instrumentation partly obliterated the dentinal tubules with a characteristic conformation similar to "flute-mouthpiece" (Fig. 2D). Nevertheless, the effect of the mild acidic solution removed a great deal of smear layer from the root surfaces treated with curettes (Fig. 3A). Closer views of the samples showed that remnant smear layer was present on the root surface and although the majority of the tubules were exposed, few of them were still occluded by smear plugs (Fig. 3B). The grade 2 acid challenge was attributed to the CRT/acid-challenge Group.
In the group of samples treated with ultrasonic device the effect the mild acidic solution was able to remove completely the smear layer from the root surface (Fig. 3C). Closer views of the samples showed that no residual debris of smear layer was present on the root surface and no smear plugs occluded the orifices of the dentinal tubules (Fig. 3D). Furthermore, some exposed collagen fibrils were also observed (Fig. 3D). The grade 1 smear layer removal was attributed to the USC/acid-challenge Group.
Subsequently to acid challenge, the samples of the two principal groups treated with phytocomplex oxalate-containing solution showed the presence of acid resistant g-calcium oxalate micro-crystals on the dentine surface and into the dentinal tubules (Fig. 4A and 4B). Figures 4C and 4D show the morphology of calcium oxalate crystals with an eight-faced bipyramid shape. The grade 3 smear layer removal was attributed to these groups.
Table 1 shows the number of exposed dentinal tubules in each different subgroup.
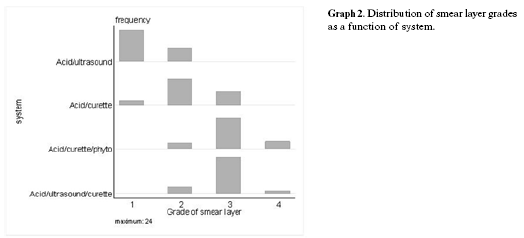
Statistical analyses showed that the phytocomplex treatment was statistically significant (p = 0.001) when compared to the acid treated groups; the pair wise comparisons are summarised in Table 2 and Graph 1. The distribution of smear layer grades is shown in Table 2 and Graph 2. There was a significant effect between the groups (p = 0.001). An adjusted residual greater in absolute value than 2 ~ 3 indicates that the null hypothesis of independence is not applicable to the cell in Table 2 and Graph 2.
Discussion
The erosive potential of an acidic soft drink is dependent upon the immediate effect on the tooth surface (33), the drinking method (34) and the protective effect of saliva (35-37). We intended to simulate the situation in which the smear layer created on the root surface by non-surgical periodontal treatments comes in contact with acidic soft drinks and contemporarily evaluate the protection offered by an oxalate-containing phytocomplex solution in preventing dentinal tubule exposure. The smear layers formed on the root surface after hand instrumentation with the curette covered the dentine and completely occluded the dentinal tubules. Furthermore, the smear layer appeared multilayered and characterised by "tree-bark" grooves, which may be induced by stokes during the hand instrumentation.
In this case, it can be speculated that dentine hypersensitivity does not occur immediately after hand instrumentation by curette due to the presence of a smear layer that occluded the dentinal tubules. This characteristic may explain why dentine hypersensitivity typically occurs a few days later, when the smear layer has been removed by tooth-brushing, dietary substances, saliva or other endogenous factors (37). Conversely, the morphology of the root surfaces after instrumentation with ultrasonic instrumentation is characterised by the presence of grooves and by a very thin layer of debris that does not completely cover the root dentine and only partially obliterates the dentinal tubules. This is the situation in which dentine hypersensitivity might occur immediately after instrumentation.
The risk for post operative dentine hypersensitivity is increased by the pH of acidic soft drinks especially after non-surgical periodontal treatments. One of the main acids involved as a constituent of many fruit juices and acidic soft drinks is citric acid (38) with a typical concentration of [0.2-0.004 M] for many fruit juices and [0.015-0.05 M] for the soft drinks (39).
Previous studies have indicated that the critical pH for enamel dissolution is 5.5, and any solution with a lower pH may cause mineral dissolution of dental hard tissues (40, 41). Larsen and Nyvad (1999) (42) found a relation between the pH of soft drinks and the erosive potential on teeth. However, the titratable acidity was also showed to have an effect on the erosive potential (43, 36). Furthermore, Correa et al. (2004) demonstrated the acidic influence of natural fruit juices in removing the smear layer from the root surface.
In agreement with these recent studies, the results obtained in this study regarding the influence of mild acidic solutions, showed that the exposure of the root surface to a substance with low pH was associated with the opening of the dentinal tubules and with the removal of the smear layer from the root surfaces. The SEM investigation revealed that the root surfaces treated by curette instrumentation and subsequently submitted to a mild acidic challenge showed some smear debris present on the root surface. Although most tubules were exposed, some of them were still occluded by smear plugs. On the other hand, the mild acidic solution was able to remove completely the smear layer from the root surface treated with ultrasonic and no smear plugs were found to be present into the openings of the tubules. It has also been reported that application of various acidic drinks modifies dentine permeability even after brushing procedures with and without toothpaste (44). Moreover, the dentine permeability after brushing with toothpaste was significantly lower than that observed after brushing without toothpaste, which was in turn lower than that observed with a previous application of acid. According to these results, we can speculate that tooth-brushing should not immediately follow ingestion of acidic drinks but should be separated from mealtimes. Additionally, Ponduri et al., (2006) (45) showed that the combination of erosion and abrasion resulted in significantly greater loss of dentine than erosion alone. It was also demonstrated that fluoride toothpaste could provide a partial protection against erosion, which supports the concept of brushing before meals. On the other hand, the SEM results of the present study also show that treatment with oxalate-containing phytocomplex spray after non surgical periodontal treatments induces microcrystal deposition on the dentinal surface and into the dentinal tubules and reduce the tubular diameters by forming crystals or crystal-like structures. This oxalate crystal formation prevents the dissolution of smear layer and the exposure of the dentinal tubules. The morphology of calcium oxalate crystals is an eight-faced bipyramid shape (tetragonal system) which corresponds to weddellite or dehydrated calcium oxalate (CaC2O4o2H2O) (46, 47). The crystal polymorphism, defined as the formation of crystals with different shapes, seems to be associated with different environmental parameters during crystallogenesis. The soluble oxalates and oxalic acid present in the tested phytocomplex solution form calcium oxalate crystals by reacting with dentinal calcium (48). The calcium oxalate crystals already present in the lyophilised phytocomplexes may penetrate into the dentinal tubules if their dimensions are less than 2 mm. Moreover, calcium oxalate crystals are insoluble and they may bind to anionic macromolecules such as dentinal proteins (49).
It is well known that oxalates are able to create crystals, most likely calcium crystals, when applied to dentinal tissue. They produce a layer of crystals that reduces dentinal permeability. However, only a few foods are high in oxalates. In nature, for example, some common vegetables such as spinach, rhubarb and mint contain phytocomplexes that may be easily prepared and used for pastes or gels for dental hygiene (46). The solution used in this study was able to create oxalate crystals that were detectable inside tubule orifices and may be suitable for topical treatment of dentinal hypersensitivity. Further evaluations are in progress to create suitable clinical formulations suitable for commercial products.
Conclusion
This study demonstrated that the smear layer created on the root surface after non-surgical periodontal treatments may be affected and removed by acidic soft drinks. Thus, the use of acidic soft drinks immediately after periodontal therapy should be avoided to prevent any smear layer removal and dentinal hypersensitivity. However, phytocomplexes extracted from rhubarb and spinach used in the form of a 1.5% spray solution may be suitable for topical treatment after periodontal instrumentation in order to prevent smear layer dissolution and dentinal tubules exposure. The new phytocomplex solution might play a clinical role in the prevention of dentinal hypersensitivity caused by scaling and root planing procedures.
References
1. Albair WB, Cobb CM, Killoy WJ. Connective tissue attachment to periodontally diseased roots after citric acid demineralization. J Periodontol. 1982 Aug;53(8):515-26. [ Links ]
2. Aleo JJ, De Renzis FA, Farber PA, Varboncoeur AP. The presence and biologic activity of cementum-bound endotoxin. J Periodontol. 1974 Sep;45(9):672-5. [ Links ]
3. Aleo JJ, De Renzis FA, Farber PA. In vitro attachment of human gingival fibroblasts to root surfaces. J Periodontol. 1975 Nov;46(11):639-45. [ Links ]
4. Allen EF, Rhoads RH. Effect of high speed periodontal instruments on tooth surface. J Periodontol 1963; 34:352-6. [ Links ]
5. Auplish G, Needleman IG, Moles DR, Newman HN. Diamond-coated sonic tips are more efficient for open debridement of molar furcations. A comparative manikin study. J Clin Periodontol. 2000 May;27(5):302-7. [ Links ]
6. Badersten A, Nilvéus R, Egelberg J. Scores of plaque, bleeding, suppuration and probing depth to predict probing attachment loss. 5 years of observation following nonsurgical periodontal therapy. J Clin Periodontol. 1990 Feb;17(2):102-7. [ Links ]
7. Belting CM, Spjut PJ. Effects of high-speed perioodontal instruments on the rood surface during subgingival calculus removal. J Am Dent Assoc. 1964 Nov;69:578-84. [ Links ]
8. Caton J, Bouwsma O, Polson A, Espeland M. Effects of personal oral hygiene and subgingival scaling on bleeding interdental gingiva. J Periodontol. 1989 Feb;60(2):84-90. [ Links ]
9. Chace R. Subgingival curettage in periodontal therapy. J Periodontol. 1974 Feb;45(2):107-9. [ Links ]
10. Cheetham WA, Wilson M, Kieser JB. Root surface debridement--an in vitro assessment. J Clin Periodontol. 1988 May;15(5):288-92. [ Links ]
11. Chiew SY, Wilson M, Davies EH, Kieser JB. Assessment of ultrasonic debridement of calculus-associated periodontally-involved root surfaces by the limulus amoebocyte lysate assay. An in vitro study. J Clin Periodontol. 1991 Apr;18(4):240-4. [ Links ]
12. Clark SM, Grupe HE, Mahler DB. The effect of ultrasonic instrumentation on root surfaces. J Periodontol. 1968 May;39(3):135-7. [ Links ]
13. Coldiron NB, Yukna RA, Weir J, Caudill RF. A quantitative study of cementum removal with hand curettes. J Periodontol. 1990 May;61(5):293-9. [ Links ]
14. Cole RT, Crigger M, Bogle G, Egelberg J, Selvig KA. Connective tissue regeneration to periodontally diseased teeth. A histological study. J Periodontal Res. 1980 Jan;15(1):1-9. [ Links ]
15. Rossi R, Smukler H. A scanning electron microscope study comparing the effectiveness of different types of sharpening stones and curets. J Periodontol. 1995 Nov;66(11):956-61. [ Links ]
16. Fogel HM, Pashley DH. Effect of periodontal root planing on dentin permeability. J Clin Periodontol. 1993 Oct;20(9):673-7. [ Links ]
17. Chabanski MB, Gillam DG, Bulman JS, Newman HN. Clinical evaluation of cervical dentine sensitivity in a population of patients referred to a specialist periodontology department: a pilot study. J Oral Rehabil. 1997 Sep;24(9):666-72. [ Links ]
18. Taani SD, Awartani F. Clinical evaluation of cervical dentin sensitivity (CDS) in patients attending general dental clinics (GDC) and periodontal specialty clinics (PSC). J Clin Periodontol. 2002 Feb;29(2):118-22. [ Links ]
19. Prati C, Montebugnoli L, Suppa P, Valdrè G, Mongiorgi R. Permeability and morphology of dentin after erosion induced by acidic drinks. J Periodontol. 2003 Apr;74(4):428-36. [ Links ]
20. Addy M, Dowell P. Dentine hypersensitivity--a review. Clinical and in vitro evaluation of treatment agents. J Clin Periodontol. 1983 Jul;10(4):351-63. [ Links ]
21. Pashley DH. Mechanisms of dentin sensitivity. Dent Clin North Am. 1990 Jul;34(3):449-73. [ Links ]
22 Pashley DH. Dentin permeability, dentin sensitivity, and treatment through tubule occlusion. J Endod. 1986 Oct;12(10):465-74. [ Links ]
23 Prati C. What is the clinical relevance of in vitro dentine permeability tests. J Dent. 1994 Apr;22(2):83-8. [ Links ]
24. Mongiorgi R, Prati C. Mineralogical and chrystallographical study of g-calcium oxalate on dentine surfaces in vitro. Arch Oral Biol 1994;39(Suppl.):152s. [ Links ]
25. Kerns DG, Scheidt MJ, Pashley DH, Horner JA, Strong SL, Van Dyke TE. Dentinal tubule occlusion and root hypersensitivity. J Periodontol. 1991 Jul;62(7):421-8. [ Links ]
26. Gillam DG, Mordan NJ, Sinodinou AD, Tang JY, Knowles JC, Gibson IR. The effects of oxalate-containing products on the exposed dentine surface: an SEM investigation. J Oral Rehabil. 2001 Nov;28(11):1037-44. [ Links ]
27. Pashley DH, Tao L, Boyd L, King GE, Horner JA. Scanning electron microscopy of the substructure of smear layers in human dentine. Arch Oral Biol. 1988;33(4):265-70. [ Links ]
28. Morris MF, Davis RD, Richardson BW. Clinical efficacy of two dentin desensitizing agents. Am J Dent. 1999 Apr;12(2):72-6. [ Links ]
29. Holmes RP, Kennedy M. Estimation of the oxalate content of foods and daily oxalate intake. Kidney Int. 2000 Apr;57(4):1662-7. [ Links ]
30. Noonan SC, Savage GP. Oxalate content foods and its effect on humans Asia Pacific. J Clin Nutr 1999;8:64-74. [ Links ]
31. Monje PV, Baran EJ. Characterization of calcium oxalates generated as biominerals in cacti. Plant Physiol. 2002 Feb;128(2):707-13. [ Links ]
32. Pereira JC, Segala AD. In vitro effects of KOx based desensitizing agents on hydraulic conductance of human dentin submitted to different surface treatments. J Dent Res 1999;78(Suppl.):252. [ Links ]
33. Bashir E, Lagerlof F. Effect of citric acid clearance on the saturation with respect to hydroxyapatite in saliva. Caries Res. 1996;30(3):213-7. [ Links ]
34. Johansson AK, Lingstrom P, Imfeld T, Birkhed D. Influence of drinking method on tooth-surface pH in relation to dental erosion. Eur J Oral Sci. 2004 Dec;112(6):484-9. [ Links ]
35. Meurman JH, Frank RM. Scanning electron microscopic study of the effect of salivary pellicle on enamel erosion. Caries Res. 1991;25(1):1-6. [ Links ]
36. Jensdottir T, Bardow A, Holbrook P. Properties and modification of soft drinks in relation to their erosive potential in vitro. J Dent. 2005 Aug;33(7):569-75. [ Links ]
37. Correa FO, Sampaio JE, Rossa Junior C, Orrico SR. Influence of natural fruit juices in removing the smear layer from root surfaces--an in vitro study. J Can Dent Assoc. 2004 Nov;70(10):697-702. [ Links ]
38. Lussi A, Jaeggi T, Zero D. The role of diet in the aetiology of dental erosion. Caries Res. 2004;38 Suppl 1:34-44. [ Links ]
39. Barbour ME, Parker DM, Allen GC, Jandt KD. Human enamel dissolution in citric acid as a function of pH in the range 2.30< or =pH< or =6.30--a nanoindentation study. Eur J Oral Sci. 2003 Jun;111(3):258-62. [ Links ]
40. Ten Cate JM, Imfeld T. Dental erosion, summary. Eur J Oral Sci. 1996 Apr;104:241-4. [ Links ]
41. Hughes JA, West NX, Parker DM, Newcombe RG, Addy M. Development and evaluation of a low erosive blackcurrant juice drink. 3. Final drink and concentrate, formulae comparisons in situ and overview of the concept. J Dent. 1999 Jul;27(5):345-50. [ Links ]
42. Larsen MJ, Nyvad B. Enamel erosion by some soft drinks and orange juices relative to their pH, buffering effect and contents of calcium phosphate. Caries Res. 1999;33(1):81-7. [ Links ]
43. Lussi A, Jaeggi T, Jaeggi-Scharer S. Prediction of the erosive potential of some beverages. Caries Res. 1995;29(5):349-54. [ Links ]
44. Prati C, Venturi L, Valdre G, Mongiorgi R. Dentin morphology and permeability after brushing with different toothpastes in the presence and absence of smear layer. J Periodontol. 2002 Feb;73(2):183-90. [ Links ]
45. Ponduri S, Macdonald E, Addy M. A study in vitro of the combined effects of soft drinks and tooth brushing with fluoride toothpaste on the wear of dentine. Int J Dent Hyg. 2005 Feb;3(1):7-12. [ Links ]
46. Sauro S, Gandolfi MG, Prati C, Mongiorgi R. Oxalate-containing phytocomplexes as dentine desensitisers: an in vitro study. Arch Oral Biol. 2006 Aug;51(8):655-64. [ Links ]
47. Sauro S, Mongiorgi R, Chersoni S, Breschi L, Ferrieri P, Prati C. Dentine hypersensitivity treatment with phytocomplex oxalates pastes: a SEM and dentine permeability measurement study. J It Cons 2005; 4: 228-3 [ Links ]
48. Orchardson R, Gillam DG. The efficacy of potassium salts as agents for treating dentin hypersensitivity. J Orofac Pain. 2000 Winter;14(1):9-19. [ Links ]
49. Franceschi VR, Corner HT. Calcium oxalate crystals in plants. Bot Rev 1980;46: 361-427. [ Links ]
![]() Correspondence:
Correspondence:
Dr. Salvatore Sauro
Kings College London Dental Institute
Guy`s,Kings College and St Thomass Hospitals
Biomaterials and Biomimetic materials
Floor 17, Guys Tower, Guys Hospital
London, United Kindom
SE1 9RT
E-mail:salvatore.sauro@kcl.ac.uk
Received: 18-05-2007
Accepted: 21-07-2007