Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Medicina Oral, Patología Oral y Cirugía Bucal (Internet)
versión On-line ISSN 1698-6946
Med. oral patol. oral cir.bucal (Internet) vol.12 no.8 dic. 2007
Oral necrotizing microvasculitis in a patient affected by Kawasaki disease
Giuseppe Alessandro Scardina1, Gerlandina Fucà2, Francesco Carini2, Vincenzo Valenza2, Michele Spicola3, Paolo Procaccianti3, Pietro Messina1, Emiliano Maresi3
(1) Department of Oral Science-University of Palermo
(2) Department of Experimental Medicine-Section of Human Anatomy-University of Palermo
(3) Department of Pathological Anatomy-University of Palermo. Italy
ABSTRACT
Kawasaki disease (KD) was first described in 1967 by Kawasaki, who defined it as "mucocutaneous lymph node syndrome".
KD is an acute systemic vasculitis, which mainly involves medium calibre arteries; its origin is unknown, and it is observed in children under the age of 5, especially in their third year.
The principal presentations of KD include fever, bilateral nonexudative conjunctivitis, erythema of the lips and oral mucosa, changes in the extremities, rash, and cervical lymphadenopathy.
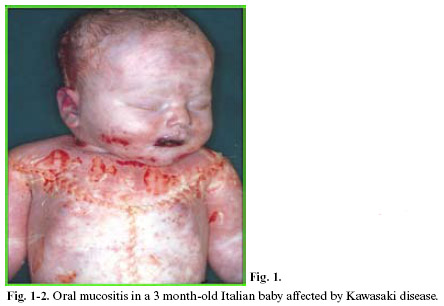
Within KD, oral mucositis – represented by diffuse mucous membrane erythema, lip and tongue reddening and lingual papillae hypertrophy with subsequent development of strawberry tongue – can occur both in the acute stage of the disease (0-9 days), and in the convalescence stage (>25 days) as a consequence of the pharmacological treatment. KD vascular lesions are defined as systemic vasculitis instead of systemic arteritis. This study analyzed the anatomical-pathological substrata of oral mucositis in a baby affected by Kawasaki disease and suddenly deceased for cardiac tamponade caused by coronary aneurysm rupture (sudden cardiac death of a mechanical type).
Key words: Kawasaki disease, oral mucositis, vasculitis.
Introduction
Kawasaki disease (KD) is an acute systemic vasculitis which mainly involves medium calibre arteries; its origin is unknown, and it affects children under the age of 5, especially in their third year (1-5).
Epidemiological studies and clinical presentation suggest an infective origin of the disease (5). Although a variety of infective agents have been proposed - rickettsiae, viruses (Epstein-Barr virus and retrovirus), viridans streptococcus, staphylococci, parvovirus and propionibacterium – no specific aetiological agent has been identified so far; therefore, the infection is deemed to be the trigger for the disease in genetically susceptible subjects (substratum) (6,7).
KD was first described in 1967 by Kawasaki, who defined it as "mucocutaneous lymph node syndrome" (8-10). KD yearly incidence in the world amounts to approximately 3.4-100 cases/100,000 people. In Japan, the incidence of the disease is 10 times higher than in the United States and 30 times higher than in Great Britain and Australia. Moreover, the Japanese immigrants maintain the incidence of their country of origin, strengthening the hypothesis of a genetic substratum (11,12). Today, KD diagnosis is formulated on the basis of a combination of distinctive clinical aspects (diagnostic criteria) and laboratory data (1).
The diagnostic criteria include: feverish state, which lasts at least 5 days and does not disappear with the usual antipyretic drugs (antibiotics etc.); polymorphous rash; conjunctival congestion; oropharyngeal mucositis (erythematous and cracked lips, strawberry tongue, pharyngeal erythema); periungual erythema, swelling and peeling on upper and lower limbs, laterocervical lymphadenitis (9,13). These aspects can be associated to irritability, meningism, diarrhoea, hepatitis, hydrops of gallbladder, urethritis, otitis media and arthritis (1).
The cardiac involvement, in the forms of myocarditis, pericarditis, coronal aneurysms, valvular failure, etc., is always present – even though in the latent form in some cases – and it is responsible for the decease from occlusive thrombosis of the typical vasculitic coronal aneurysms, which often occurs during the convalescence stage of the disease (1,14-19).
Laboratory data include leukocytosis, high ESR, positivity to C-reactive protein, thrombocytosis (1,20).
The observation of numerous patients deceased from autopsically ascertained KD, whose diagnosis in life had been delayed or even not formulated due to the absence of some of the 5 diagnostic criteria, has permitted the identification of "atypical" or "incomplete" forms of KD (21,22). The latter are observed in children under the age of 2, and mainly in babies (<6 months). In atypical KD (AKD) forms, the most frequently missing symptoms are: cervical lymphadenopathy, mucositis and polymorphous exanthema. On the other hand, in AKD, all laboratory data are always present (23,24).
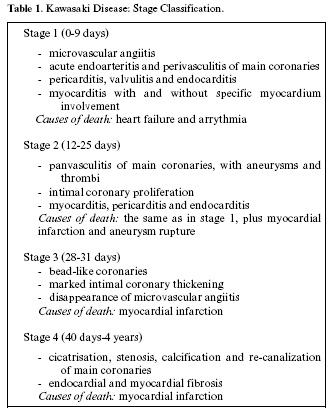
The clinical course of KD can be divided into 3 clinical stages (Table 1): acute, subacute and convalescence (1). The acute feverish stage usually lasts from 7 to 14 days (after which temperature returns to normal), and is characterized by conjunctival congestion, oropharyngeal mucositis, periungual erythema and peeling, polymorphous rash and laterocervical lymphadenopathy. The subacute stage includes the period between the end of the feverish and the 25th day of the disease. During this stage, the patients show skin peeling on the limbs, arthritis, arthralgia and thrombocytosis. The convalescence stage begins when the clinical symptoms start disappearing, and continues till ESR normalization, which is observed after 6 to 8 weeks (1,25).
Anatomical-pathological studies have shown how the clinical manifestations of KD depend on specific morphological substrata detectable at the cardiovascular level, which, according to their chrono-histogenesis, have permitted the formulation of an accurate stage classification (14).
KD is very rare in non Oriental children having less than 6 months.
This study analyzed the anatomical-pathological substrata of oral mucositis in a 3 month-old Italian baby affected by Kawasaki disease and suddenly deceased for cardiac tamponade caused by coronary aneurysm rupture (sudden cardiac death of a mechanical type).
Case Report
This case regards a 3 month-old Italian male baby who, three days after the compulsory vaccination (I dose of antipolio, anti-hepatitis B, anti-diphteric and antipneumonia vaccination) and antipyretic administration (Lonarid®) to calm the feverish condition caused by this, gradually showed eyelid conjunctival and lip erythema, diffuse cutaneous exanthema and pharingo-tonsillitis associated to crises of staring which lasted for a few seconds. For the latter reason, the baby was hospitalized in the Pediatrics and Neonatology Division. At admission, his temperature was 39°C; leukocytes 7,500; transaminases 100 (GOT), 79 (ALT), 192 (Gamma-GT).
On the following day, given the unvaried clinical situation, which mainly showed high temperature and multiarea erythemas, the baby was transferred to the Infectious Disease Division for suspected sepsis. At admission, he underwent antibiotic therapy (Rocefin®) associated to cortisone (Bentelan®-1mg-die) for the presence of diffuse hoarse breathing with oropharyngitis; the haemato-chemical data were: erythrocytes 3.51x10e6/uL; leukocytes 9.92x10e3/uL; platelets 626x10e3/uL; alcaline phosphatase 334 IU/L, PCR 7.85mg/dl; ALT 49IU/L. The other clinical manifestations described above remained unchanged. Further haemato-chemical tests excluded the presence of infections due to cytomegalovirus, toxoplasma, hepatitis virus and listeria; an abdominal ecography was carried out, which merely revealed a hepatosplenomegaly with an even parenchymal structure. On the third day after admission, due to the appearance of skin pallor, pained expression, perioral cyanosis, alae nasi agitation, medium bubble rales at the right middle pulmonary lobe, presence of skin fovea at the palpation of the lower limbs, an echocardiogram was carried out, which showed: diffusely hyperechogenic right coronary, dilated in the proximal tract with 4 mm aneurismal margin; hyperechogenic left coronary, dilated at the level of the common trunk, with aneurysmatic aspect at the level of the first tract of anterior interventricular septum (AIV); moderate pericardial effusion; widely preserved myocardial contractility. At that stage, laboratory tests showed: erithrocytes 2.85x10e3/uL; leukocytes 33,35x10e3/uL, neutrophiles 71.5%; platelets 297x10e3/uL; haemoglobin 8.7 g/dL; prothrombin activity 50%; antithrombin III 74%; myeloreticular ratio 12-13; well-represented megakariocytes; absence of blast cells. Culture exams did not show the presence of salmonella, shigella, etc.
Decease occurred nine days after admission, from sudden cardiac arrest, unresponsive to any type of intensive care treatment.
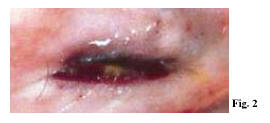
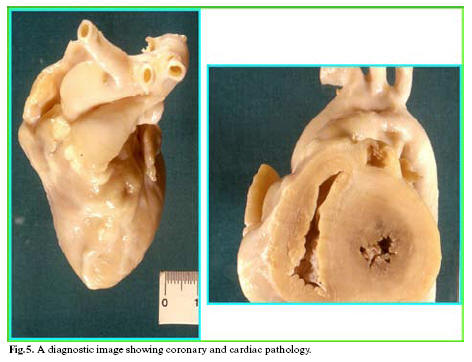
The systematic autopsy revealed that the cause of death was due to inflammatory aneurysm rupture in the anterior descending coronary, with subsequent cardiac tamponade (sudden cardiac death of a mechanical type), and that numerous foci of necrotizing microvasculitis with mixed granulo- lympomonocytic infiltrate, which mainly involved the arteriole-capillary area, were present at both cutaneous and mucosal levels (oropharyngeal mucous membrane). The anatomical-pathological and clinical outline suggested a typical Kawasaki disease (Figs 1-5).


Discussion
Within Kawasaki disease (KD), oral mucositis – represented by diffuse mucous membrane erythema, lip and tongue reddening and lingual papillae hypertrophy with subsequent development of strawberry tongue – can occur both in the acute stage of the disease (0-9 days), and in the convalescence stage (>25 days) as a consequence of the pharmacological treatment. Oral mucositis depends on the typical necrotizing microvasculitis with fibrinoid necrosis, which is common in Kawasaki disease and connected to an involvement of mucous-cutaneous and visceral systemic areas (conjunctival and pharyngeal congestion, polymorphous rash, etc.).
The key pathological evidence of Kawasaki disease (KD) is a multi-systemic vasculitis, which mainly involves medium calibre arteries (4). Such an involvement is particularly relevant because it affects the main coronaries leading to the development of aneurysms, which can determine decease through the formation of occlusive thrombi or wall rupture, with cardiac tamponade (1,16,19,26). Post-mortem morphological studies have shown how the vascular lesions vary according to the duration of the disease and the time of death. Vasculitis mainly involves microvessels (arterioles, capillaries and venules) and small arteries, at the level of both the adventitia of medium calibre muscular-elastic arteries (perivasculitis), and the systemic connective tissue (microvasculitis) (6). Perivasculitis is responsible for the subsequent phlogistic involvement of the tunica media of muscular-elastic arteries, and therefore for the development of aneurysm, whose usual site is the subpericardial coronary arterial bed (15).
Necrotizing microvasculitis (fibrinoid necrosis) is observed in the systemic connective tissue at the cardiac level, but mainly at the cutaneous and at the mucosal levels, and represents the typical morphological substratum of mucous-cutaneous and visceral clinical manifestations of the acute stage of the disease. For this reason, KD vascular lesions are defined as systemic vasculitis instead of systemic arteritis.
In the acute stage of the disease, circulation shows cytotoxic antibodies for the endothelial cells prestimulated by exposure to various cytokines (20).
Circulating cytotoxic antibodies directed towards components of neutrophil cytoplasm (ANCA, antineutrophil cytotoxic antibodies), which are observed in other forms of vasculitis, such as PAN and Wegeners granulomatosis, were also observed in about one third of the children during acute KD (20,26).
There is no specific diagnostic assay for KD; therefore, diagnosis is based on clinical criteria, which include fever for at least five days and four or more of the five major clinical features (i.e., conjunctival injiection, cervical lynphadenopathy, oral mucosal changes, polymorphous rash, and swelling or rednessof the extremities), and exclusion of alternative diagnoses. Clinical features may not be present simultaneously, and taking a careful history is necessary in children who lack a clear explanation for fever. If the typical clinical findings are present in a child with fever for less than five days, the diagnosis still can be made by experienced physicians. Because many of the clinical features of KD may be present in other illness, exclusion of other illness (toxic shock syndrome, Stevens-Johnson syndrome, measles, scarlet fever, drug reactions, other febrile viral exanthemas, juvenile rheumatoid arthritis, leptospirosis, mercury poisoning) in the differential diagnosis often is necessary (27-30).
In the case described the diagnosis was made after the death, due to the absence of some of the diagnostic criteria, moreover the oral-pharyngeal signs have been probably under evaluated (AKD).
The case of oral mucositis described in this study confirm literature data, revealing the morphological substratum constituted by microcirculation-necrotizing vasculitis.
References
1. Maresi E, Passantino R, Midulla R, Ottoveggio G, Orlando E, Becchina G, et al. Sudden infant death caused by a ruptured coronary aneurysm during acute phase of atypical Kawasaki disease. Hum Pathol. 2001 Dec;32(12):1407-9. [ Links ]
2. Limbach HG, Lindinger A. Kawasaki syndrome in infants in the first 6 months of life. Klin Padiatr. 1991 May-Jun;203(3):133-6. [ Links ]
3. Hamashima Y, Furukawa F, Kao T, Shimizu J. Kawasaki disease and mites. Nippon Rinsho. 1983 Sep;41(9):2023-8. [ Links ]
4. Kato H, Sasaguri Y, Morimatsu M. Kawasaki disease and polyarteritis of infants. Ryumachi. 1983 Oct;23(5):376-81. [ Links ]
5. Rubin B, Cotton DM. Kawasaki disease: a dangerous acute childhood illness. Nurse Pract. 1998 Feb;23(2):34, 37-8, 44-8. [ Links ]
6. Hamashima Y, Takasaka K, Fujiwara H. Kawasakis disease-its pathological features and possible pathogenesis. Acta Paediatr 1983; 42: 108-17. [ Links ]
7. Bradley DJ, Glodé MP. Kawasaki disease. The mystery continues. West J Med. 1998 Jan;168(1):23-9. [ Links ]
8. Ahlström H, Lundström NR, Mortensson W, Ostberg G, Lantorp K. Infantile periarteritis nodosa or mucocutaneous lymph node syndrome. A report on four cases and diagnostic considerations. Acta Paediatr Scand. 1977 Mar;66(2):193-8. [ Links ]
9. Kato H, Koike S, Yamamoto M, Ito Y, Yano E. Coronary aneurysms in infants and young children with acute febrile mucocutaneous lymph node syndrome. J Pediatr. 1975 Jun;86(6):892-8. [ Links ]
10. Melish ME. Kawasaki syndrome (the mucocutaneous lymph node syndrome). Annu Rev Med. 1982;33:569-85. [ Links ]
11. Amano S, Hazama F, Hamashima Y. Pathology of Kawasaki disease: II. Distribution and incidence of the vascular lesions. Jpn Circ J. 1979 Aug;43(8):741-8. [ Links ]
12. Fukushige J, Takahashi N, Ueda Y, Ueda K. Incidence and clinical features of incomplete Kawasaki disease. Acta Paediatr. 1994 Oct;83(10):1057-60. [ Links ]
13. Kato H, Nishiyori A, Sugimura T, Sasaguri Y. Kawasaki vasculitis. Nippon Rinsho. 1994 Aug;52(8):2095-102. [ Links ]
14. Fujiwara H, Hamashima Y. Pathology of the heart in Kawasaki disease. Pediatrics. 1978 Jan;61(1):100-7. [ Links ]
15. Amano S, Hazama F, Hamashima Y. Pathology of Kawasaki disease: I. Pathology and morphogenesis of the vascular changes. Jpn Circ J. 1979 Jul;43(7):633-43. [ Links ]
16. Behr E, Wood DA, Wright M, Syrris P, Sheppard MN, Casey A, et al. Cardiological assessment of first-degree relatives in sudden arrhythmic death syndrome. Lancet. 2003 Nov 1;362(9394):1457-9. [ Links ]
17. Sasaguri Y, Kato H. Regression of aneurysms in Kawasaki disease: a pathological study. J Pediatr. 1982 Feb;100(2):225-31. [ Links ]
18. Takahashi M, Mason W, Lewis AB. Regression of coronary aneurysms in patients with Kawasaki syndrome. Circulation. 1987 Feb;75(2):387-94. [ Links ]
19. Lie J, Sanders J. Sudden death in early infancy from accelerated late sequalae of coronary artery aneurysms. Cardiovasc Pathol 1997; 6:175-8. [ Links ]
20. Fujimoto T, Kato H, Ichiose E, Sasaguri Y. Immune complex and mite antigen in Kawasaki disease. Lancet. 1982 Oct 30;2(8305):980-1. [ Links ]
21. Rossomando V, Baracchini A, Chiaravalloti G, Assanta N, Buti G, Matteucci L, et al. Atypical and incomplete Kawasaki disease. Minerva Pediatr. 1997 Sep;49(9):419-23. [ Links ]
22. Huang YC, Huang FY, Lee HC. Atypical Kawasaki disease: report of two cases. Zhonghua Min Guo Xiao Er Ke Yi Xue Hui Za Zhi. 1992 May-Jun;33(3):206-11. [ Links ]
23. Balz D, Arbents V, Fanconi S. Myocarditis and coronary dilation in the 1st week of life: Neonatal incomplete Kawasaki disease? Eur J Pediatr 1998; 157:589-91. [ Links ]
24. Hsieh YC, Wu MH, Wang JK, Lee PI, Lee CY, Huang LM. Clinical features of atypical Kawasaki disease. J Microbiol Immunol Infect. 2002 Mar;35(1):57-60. [ Links ]
25. Melish ME, Hicks RV, Reddy V. Kawasaki syndrome: an update. Hosp Pract (Hosp Ed). 1982 Mar;17(3):99-106. [ Links ]
26. Hirose S, Hamashima Y. Morphological observations on the vasculitis in the mucocutaneous lymph node syndrome. A skin biopsy study of 27 patients. Eur J Pediatr. 1978 Aug 17;129(1):17-27. [ Links ]
27. López-Sánchez A, Guijarro Guijarro B, Hernández Vallejo G. Human repercussions of foot and mouth disease and other similar viral diseases. Med Oral. 2003 Jan-Feb;8(1):26-32. [ Links ]
28. Vicente Barrero M, Knezevic MR, Castellano Reyes JJ, Díaz Iglesias JM, Baez Marrero O. Kikuchi-Fujimoto disease. A case report. Med Oral. 2001 Aug-Oct;6(4):263-8. [ Links ]
29. Sabater Recolons MM, López López J, Rodríguez de Rivera Campillo ME, Chimenos Küstner E, Conde Vidal JM. Buccodental health and oral mucositis. Clinical study in patients with hematological diseases. Med Oral Patol Oral Cir Bucal. 2006 Nov 1;11(6):E497-502. [ Links ]
30. Sepúlveda E, Brethauer U, Rojas J, Fernández E, Le Fort P. Oral ulcers in children under chemotherapy: clinical characteristics and their relation with Herpes Simplex Virus type 1 and Candida albicans. Med Oral Patol Oral Cir Bucal. 2005 Apr 1;10 Suppl 1:E1-8. [ Links ]
![]() Correspondence:
Correspondence:
Dr. Scardina Giuseppe Alessandro
University of Palermo
Department of Oral Science "G.Messina"
via Del vespro,129 90127 Palermo, Italy
Email: alescard@msn.com
Received: 6-01-2007
Accepted: 1-08-2007