Dear Editor:
The World Health Organization currently classifies the use of tobacco as a mental and behavioral disorder, and the smoker as a patient who needs specific treatment2. This is an important aspect as smoking is the leading preventable cause of death worldwide and it is considered the main risk factor for cancer of lung, larynx, oral cavity, pharynx and esophagus, as well as cardiovascular and pulmonary chronic diseases3.
An effective smoking treatment includes monitoring and counseling by a multidisciplinary team associated with pharmacotherapy. Several studies suggest that direct counseling associated with pharmacotherapy is more effective for motivating smokers to stop smoking than one of these approaches alone3.
In 2005 an anti-smoking group (ASG) initiated activities offering smoking cessation treatment to employees of a university in São Paulo city and to the population in the neighborhood of the university. The group is a multidisciplinary team consisting of physicians, psychologists, nurses and pharmacists and has been acting in prevention, awareness, guidance and treatment of smoking. The treatment consisted of four weekly group meetings with one hour long per session followed by individual consultation with the physician and the pharmacist. At the first meeting a pharmaceutical lecture was provided highlighting the role of the drug in smoking cessation treatment, which medications were provided by the program, and how to properly store them.
In the first four weeks, nursing staff measured the carbon monoxide (CO) level in expired air to validate cigarette consumption. In the first pharmaceutical counseling drugs used by patient were evaluated and potential drug interactions with the pharmacotherapy, strengthening the guidance given during the lecture. Folders with explanatory text complement the oral guidance and patients were asked to report how they have used the drug, correcting potential problems related to drugs and increasing the safety of treatment.
To analyze the potential adverse events of smoking cessation and drug therapy efficacy and safety, a study was conducted and patients assisted by ASG were included since they attended the first four weekly meetings and used Nicotine Replacement Therapy (NRT) medication. The period of study was the four meetings of five groups starting treatment in October 7th 2013, November 11th 2013, February 2nd 2014, March 7th 2014 and April 14th 2014.
During this period 101 patients were attended by a pharmacist for counseling, but only 39 of them attended the four initial meetings: 24.75% attended only the first week, 18.81% two weeks, 17.82% three weeks, and 38.61% four weeks.
The most frequent comorbidities observed were anxiety (30.77%), hypertension (23.01%), gastritis and depression (20.51%). The most frequently used medications were hydrochlorothiazide, metformin, and simvastatin (7.69%) and omeprazole (10.21%) Table 1.
Table 1. Parameters used for assessment of patient clinical profiles assisted by the ASG
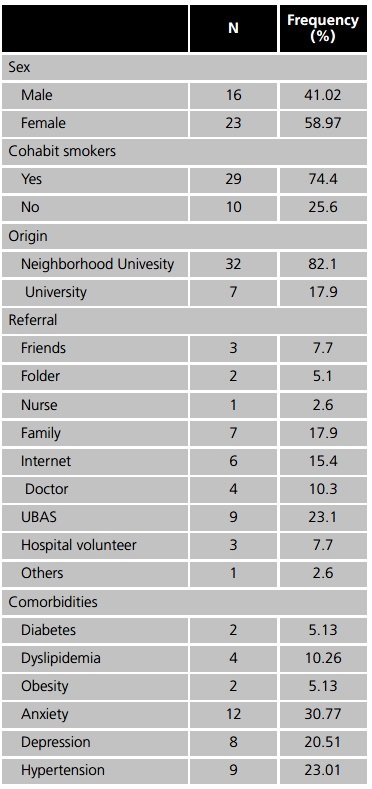
N: number of patients.
Regarding the use of NRT medicines, 79.49% of patients used only nicotine patches, 10.26% patch and nicotine gum, 7.69% nicotine gum, patch and bupropion, and 2.56% used bupropion and nicotine patch .
Smoking cessation was observed in 34.2% of patients, and among those who continue smoking, the average of cigarettes smoked reduction was 13.08 units. About the CO in expired air, 32.3% had zero levels at the fourth week, and the others showed an average reduction of 6.35 ppm.
The most common adverse reactions (ADR) observed were rash and insomnia (30.77% each) and headache (10.26%), followed by dry mouth and bitter taste (5.77% each), abnormal dreams and muscle pain at the application site (2.56% each). The majority of patients (46.15%) showed no adverse reaction. Rash and insomnia were listed as side effects in the package leaflet and in literature searching, being that the same prevalence of insomnia was described by other authors3. Patients with rash was guided to apply the patch to a different site every day and remove any glue residue from the adhesive using body oil, and those who had insomnia were instructed to not using adhesive overnight. Some patients reported dry mouth, but they used nicotine patch and gum, and possibly this ADR was due to the gum. Patient was instructed for not eating 15 minutes before and 15 minutes after taking medicines to minimize ADR, as absorption of nicotine decreased with foods that elevate pH in oral cavity. Two patients reported headache, but this might be related to the withdrawal syndrome and not to the use of medicine as demonstrated in studies comparing patients treated with NRT and placebo3.
The frequency of respiratory diseases (30.77%) corroborates the importance of investing in preventive healthcare program to assist smokers with these conditions, which represent a significant portion of Brazilian Health System costs1.
In the analysis of effectiveness of the program, 33.33% of patients were able to stop smoking within the first four weeks of treatment, similar value obtained from other quitting smoking support groups4. The CO values in expired air were used to validate the cigarette consumption reduction reported by patient, and the value of the linear correlation coefficient, 0.769, indicated a high statistical correlation. However, this finding may reflect the fact that patients with zero CO value may have not smoked before performing the analysis, since the half-life of CO in exhaled air is approximately 8 hours, and its values normalize after 4 to 8 hours of last cigarette consumed. There is also evidence that the amounts of CO are directly related to smoking duration, and degree of nicotine dependence5.
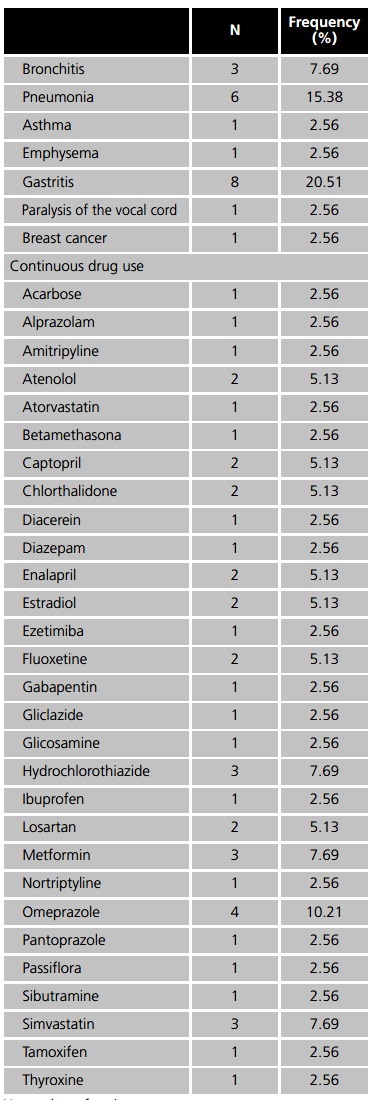
The drugs for continuous treatment most frequently used among patients were omeprazole (10.21%), hydrochlorothiazide, metformin and simvastatin (7.69% each), however, none of them have interactions with the medications dispensed by the ASG, so that no pharmaceutical intervention was required. Moreover, smoking caused pharmacokinetic alterations in some of the medications, such as alprazolam, diazepam (2.56% each) and estradiol (5.13%), because of this, patients were informed about these interactions and how it could influence the benefits of the pharmacotherapy6.
The data analysis made evident the relevance, the effectiveness and the safety of a specific approach combined with pharmacotherapy in a smoking cessation group. Moreover it showed the need for health education programs to encourage short-term smokers and young people to seek for stop smoking support groups, thus minimizing the possibility of developing smoking-related diseases.














