INTRODUCTION
Acute kidney injury (AKI) is defined as an abrupt increase in serum creatinine and/or a reduction in the rate of diuresis1. Several mechanisms are involved in AKI development, including changes in systemic and renal haemodynamics, blood pressure, volemia, renal blood flow, and glomerular filtration rate2. Risk factors for its development include both exposure to nephrotoxic drugs and patients' conditions (chronic cardiac diseases, diabetes mellitus, advanced age, and cancer, among others)1. AKI in turn contributes to the development of chronic kidney disease, cardiovascular events and reduced quality of life, increasing mortality risk3,4.
Published data on the prevalence of AKI are discordant, ranging from less than 1% to 66%, which may be due to differences in diagnostic criteria and characteristics of studied populations, among other factors5. It is estimated that 13.3 million people worldwide suffer AKI annually, with 1.7 million deaths due to AKI per year6. Mortality associated with AKI is around 24% in adults7. In critically ill patients, the risk can reach 80%2. In addition, AKI is associated with an increase in hospital stay and a greater resource consumption8,9.
The use of nephrotoxic drugs contributes to the risk of AKI, and this risk could be higher with the concomitant use of several drugs that affect kidney function. This situation is frequent in patients with multiple pathologies. In this regard, the simultaneous administration of diuretics, antihypertensives that inhibit the renin-angiotensin system [Angiotensin Converting Enzyme Inhibitors (ACEI), Angiotensin II Receptor Blockers (ARB) or aliskiren], and non-steroidal anti-inflammatory drugs (NSAIDs), known as "triple whammy" (TW), has been associated with development of AKI10.
A retrospective study carried out using data from United Kingdom showed that TW was associated with a 31% increased risk of AKI, being the risk higher (82%) in the first 30 days11. A nested case-control study including 78,379 patients aged 30 years or older treated with renin-angiotensin system inhibitors and/or diuretics found that TW exposure increased AKI risk by 64% [relative risk 1.64; 95% confidence interval (95%CI) 1.25-2.14]12. According to a retrospective study carried out in Spain, the incidence of AKI was 8.82 cases/1,000 patients exposed to TW/year (95%CI 4.48-17.31), with a mortality rate of 40.3% at 12 months13.
Many of the studies related to TW include data generated in a single region in Spain13,14, whereas the present study will integrate data from the majority of the regions in Spain, being representative of the Spanish population. This approach will allow having a significantly large sample size than in previous studies, generating more solid evidence.
Metamizol is widely use in Spain for pain management. However, to date limited safety data on metamizol have been published, and there is no evidence to show that this drug produces less impairment of kidney function compared to NSAIDs. In addition, no studies have been carried out to determine the risk of AKI associated with metamizol when it is used concomitantly with diuretics and antihypertensive drugs. Therefore, it is of particular interest to determine the effect of metamizol on kidney function, and this study will generate new evidence on this issue.
The study aims to analyse whether the exposure to the TW combination versus non-exposure to TW is associated with an increased risk of hospitalization due to AKI. Secondarily, the risk associated with the TW combination that includes metamizol and the risk associated with the TW including a NSAID will be analysed separately, taking the non-exposure to TW as reference. In addition, the risk of hospitalization due to AKI depending on the moment and duration of the TW exposure will be assessed. The requirement of renal replacement therapy and mortality during hospitalization due to AKI will also be compared between people exposed and those not exposed to TW.
METHODS
Study design and source of data
A population-based case-control study nested in a cohort will be carried out. Data for the study will be extracted from the Spanish Database for Pharmacoepidemiological Research in Primary Care (BIFAP), which is managed by the Spanish Agency for Medication and Healthcare Products15. At this moment, the database includes more than 13 million anonymized medical records from the majority of regions in Spain. BIFAP mainly integrates clinical data generated in the Primary Care setting, and from some regions it also includes data from the hospital setting.
Study cohort and follow-up
The cohort will consist of adults with at least one prescription of a diuretic or an antihypertensive drug (ACEI, ARB or aliskiren) between 01/01/2009-31/12/2018. The date of the prescription of any of these drugs will be considered as the date of the cohort entry. Subjects have to be at least 18 years old at cohort entry and must have at least one year of follow-up in BIFAP database prior to cohort entry. Patients with a history of cancer, except basal cell carcinoma, will be excluded.
Subjects will be followed up until the first hospitalization due to AKI occurs during the study period (01/01/201031/12/2018), the occurrence of cancer (except basal cell carcinoma), death from any cause, loss to follow-up, or study termination (31/12/2018), whichever occurs first.
Patients suffering a hospitalization due to AKI during the study period will be considered potential cases. Each case will be matched with up to 10 randomly selected controls of the same age, sex, and region in Spain, and according to the calendar year of registration in BIFAP database. Controls must not have suffered hospitalization due to AKI on the index date corresponding to their case.
Definition of the main event
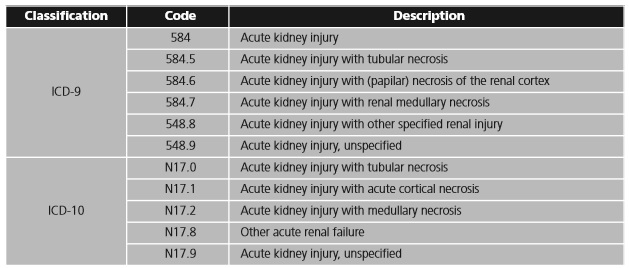
The first hospitalization due to AKI during the follow-up period will constitute the main event, and the date on which this hospitalization occurs will be considered the index date. The Ninth and Tenth Revisions of the International Classification of Diseases (ICD-9 and ICD-10) will be used to identify cases of hospitalization in which AKI figures as the main or secondary diagnosis of hospitalization (table 1).
Validation of potential cases
A validation of identified potential cases will be performed by members of the research team. This process will be carried out by selecting a randomly selected sample of cases. The medical record of these patients will be revised using the BIFAP platform. The investigators involved in this activity will review the medical records blinded to the patients drug exposure in order to avoid potential bias. Any detected discrepancies will be solved by consensus. The results of the validation process will be provided along with the research findings.
Drug exposure
The prescription of TW at any moment during the 12 months prior to the index date will represent the main exposure. The TW combination will be considered as the simultaneous prescription of at least: a) A diuretic; b) An antihypertensive drug (ACEI, ARB or aliskiren); and c) A NSAID or metamizol. Only those cases and days in which simultaneous exposure to the three components takes place will be considered as TW exposure.
The duration of a prescription will be considered to be 30 days unless otherwise stated. A treatment course will be considered continuous when there is a gap of no more than 60 days between the date of the prescription and the last dose of the previous prescription.
According to the moment of TW exposure, subjects will be classified into three categories: "current user" (exposed to TW in the 30 days prior to the index date); "recent user" (exposed to TW between 31 days and 3 months before the index date) and "past user" (exposed to TW between more than 3 months and up to 12 months before the index date). Patients without exposure to TW at any time in the 12 months prior to the index date will be considered "never users".
Two situations will be established in order to assess the risk of hospitalization due to AKI according to the duration of TW exposure. "Continuous duration" will be defined as the number of days exposed to TW continuously without interruptions, and "cumulative duration" as the total number of days exposed to TW, regardless of whether it was continuous or not.
Sample size estimation
Assuming that 11% of the controls may have been exposed to TW, it will be necessary to include 1,525 cases (which will be matched with up to 10 controls) to detect an Odds Ratio (OR) of hospitalization due to AKI of at least 1.3, with a 90% power and an alpha risk of 5%11.
Analysed variables and data analyses
Information related to participants baseline characteristics such as age, sex, presence of comorbidities and exposure to TW and to other drugs than can affect kidney function in the 12 months prior to the index date will be extracted. Analysed comorbidities include chronic kidney disease, cardiovascular diseases, cerebrovascular diseases and chronic liver disease. Information on the patients alcohol and smoking habits and substance abuse will be also explored. Regarding to the outcome variables, the occurrence of hospitalization due to AKI will be identified using internationally established diagnostic codes (ICD-9 and ICD-10). Among those participants who suffer from hospitalization due to AKI, the requirement of renal replacement will be examined using the corresponding ICD-9 and ICD-10 codes. In addition, in the case of patients who died during the hospitalization due to AKI, this date will be registered.
Characteristics of cases and controls will be compared. Categorical variables will be described as frequency distribution, and quantitative variables as mean and standard deviation or median and interquartile range, as appropriate. Comparison of categorical variables between cases and controls will be carried out using Chi-square or Fisher's test, and in the case of continuous variables using Student's t-test or Mann-Whitney test, depending on their normality.
The main analysis will compare the risk of hospitalization due to AKI among those exposed to TW versus those not exposed to TW. In addition, the risk of hospitalization due to AKI will be analysed separately with a) the TW that includes metamizol and b) the TW that includes a NSAID, using in both cases the patients not exposed to TW as comparator.
The risk of hospitalization due to AKI will be determined depending on the moment of TW exposure ("current user", "recent user" and "past user"), and the duration of the exposure (≤3 months, >3 and ≤6 months, >6 and ≤9 months, and >9 months and (≤12 months), compared to no exposure to TW.
With regard to the cases, the requirement of renal replacement therapy and mortality during hospitalization will be compared among those patients exposed to TW and those non-exposed to TW.
The association between the TW exposure and the outcome variables will be evaluated using multivariate conditional logistic regression models. Analyses will be adjusted using relevant patients comorbidities and exposure to drugs than can affect kidney function in the 12 months prior to the index date. Results of both the crude models and the adjusted models will be presented using OR, adjusted OR and 95%CI.
A subgroup analysis will be performed to determine the risk of hospitalization due to AKI with the exposure to TW in patients older than 75 years. A sensitivity analysis restricting to cases of hospitalization in which AKI figures as the main diagnosis of hospitalization will also be carried out.
Ethics and dissemination
The study protocol was approved by the Ethics Committee of Navarre (Spain) (Pyto2019/87) and by the BIFAP scientific committee. The protocol is registered in The European Union electronic Register of Post-Authorisation Studies (EU PAS Register) of the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP) (EUPAS35832)16.
Data for the study will be extracted exclusively from BIFAP. All the information contained in this database is anonymized, not allowing the identification of the subjects. Data extraction from the BIFAP database will be carried out by its managers and by study investigators following the study protocol. All the data will be treated according to data protection regulation applicable in Spain. Obtained data will not be used for purposes other than those included in the study protocol.
The results of the investigation will be published in medical journals following The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for reporting observational studies17.
DISCUSSION
Despite the already known effect of diuretics, antihypertensive drugs that inhibit the renin-angiotensin system and NSAIDs in kidney function, the impact of the simultaneous use of these drug classes on the development of AKI needs to be analysed with greater precision using databases with a higher power that those used in previous studies, including representative population with diverse clinical conditions. The proposed approach will guarantee the validity of the findings and will therefore allow generating more solid evidence.
One of the main limitations of the study is based on the possibility of bias in relation to the categorization of the participants according to their drug exposure status. This may be due to the fact that NSAIDs and metamizol can be acquired in community pharmacies without a medical prescription, and therefore such information will not be registered in the patients medical records. However, it can be expected that this circumstance will be equally distributed both in cases and controls.
In the hospital setting, patients diagnoses are registered by the responsible healthcare professionals, and this information is then routinely validated by professionals dedicated to the coding of hospital clinical data. Therefore, there is a high degree of confidence on the validity of the categorization of the patients as cases or controls. However, as additional guarantee, an individual validation of a randomly selected sample of cases will be carried out by the research team in a blinded manner. In addition, the multivariable conditional logistic regression models that will be carried out to adjust the results by several clinical conditions and concomitant pharmacological treatments, will guarantee that the research findings are not biased by factors other than exposure to the TW combination.