INTRODUCTION
The nosocomial infections by carbapenem-resistant Enterobacteriaceae (CRE) has increased in recent years. In some countries, this situation has become a public health problem due to these bacteria are now endemic1. To address this problem, the World Health Organization (WHO) published Global Action Plan on Antimicrobial Resistance in 20152.
Most prevalent bacteria are included in Enterobacteriaceae family, such as Klebsiella pneumoniae, and Escherichia spp; or Pseudomonadaceae family such as Pseudomonas spp3. Klebsiella pneumoniae carbapenemase (KPC), Verona integrin-encoded metallo-β-lactamase (VIM) and β-lactamase OXA-48 are among the most frequent carbapenemases related with antibiotic resistance4.
The main treatment for this kind of bacteria has been beta-lactam antibiotics and carbapenem antibiotics, nevertheless, beta-lactam resistance limits treatment options for this complicated infections5.
Ceftazidime is a third generation cephalosporin against Enterobacteriaceae and Pseudomonas spp. Avibactam, the first non-beta-lactam beta-lactamase inhibitor, has in vitro activity against class A (such as KPC), class C and some class D carbapenemases (such as OXA-48). It has no activity against metallo-beta-lactamases (MBL), New Delhi metallo-β-lactamase (NDM) or VIM6,7.
Ceftazidime/avibactam (CZA) combination was approved 24 June 2016 by European Medicines Agency (EMA) for treatment of complicated intra-abdominal infection (cIAI), complicated urinary tract infection (cUTI), including pyelonephritis, hospital-acquired pneumonia (HAP), including ventilator associated pneumonia (VAP) and infections due to aerobic Gram-negative organisms with limited treatment options.
There are not many clinical practice data due to recently CZA commercialization. In vitro activity against Enterobacteriaceae is between 67.0-99.0%6. Therefore, it is important to determinate CZA susceptibility of the isolated bacteria and identify carbapenemase or beta-lactamase enzyme related to antibiotic resistance. A low rate of resistance development to CZA is reported, varying from 1.6-2.0%8,9.
Clinical response varies among 55.0-95.0%3,10 and microbiological response among 55.6-91.1%8,9,11-13. 30 day all-cause mortality rates ranges between 5.9-39.5%3,10.
Complicated infection and comorbidities might reduce clinical and microbiological response to CZA. Therefore, concomitant antibiotic against isolated bacterium might be necessary. Nevertheless, increased response to concomitant treatment is not demonstrated versus CZA monotherapy3, 6,14. Infection recurrence after CZA treatment varies among 8.7-21.6%15,16.
Therefore, nosocomial infections, resistance and the developing of new antibiotics such CZA are increasing, we designed this study in clinical practice. The main outcome was to assess the effectiveness (defined as clinical and microbiological cure) and safety profile of CZA in clinical practice. The secondary objectives were to assess the effectiveness between subgroups of patients (patients with bacteremia, Intensive Care Unit (ICU) admission and concomitant antibiotic treatment), and to assess recurrence of infection.
MATERIALS AND METHODS
Study design
This was a retrospective observational study between January 2016 and October 2018. The inclusion criteria were: 1) age ≥18 years, and 2) receipt of >24 hours of CZA. After treatment had been finished, a monitoring period of ninety days was performed. Approval was obtained by the Clinical Research Ethics Committee of the University Hospital of La Princesa.
Variables and definitions
All patient data were collected from electronic history records and electronic prescribing system. Variables studied included demographic (age and sex), clinical (admission unit, Charlson Index of co-morbidities at admission and estimated glomerular filtration rate (EGFR) calculated by Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI)), microbiological (infection source or focus/foci, isolated bacteria, carbapenemase and/or beta-lactamase presence, bacteremia and ICU admission), and treatment (days until CZA treatment starting, CZA treatment duration, exceeded recommendations of time treatment by infection onset, treatment beginning causes, CZA dosage and loading dose (4g of ceftazidime and 2g of avibactam), CZA adjustment dose by EGFR (≤50 ml/min), previous systemic antibiotic use, concomitant systemic antibiotic use, CZA susceptibility (by the European Committee on Antimicrobial Susceptibility Testing (EUCAST) antimicrobial susceptibility testing regulations), adverse events, causes of treatment discontinuation, clinical and microbiological cure, infection recurrence evaluated at 90 days, CZA treatment of the recurrence evaluated at 90 days and all-cause mortality during 14 and 30 days after starting CZA). Comorbidity burden was quantified using the Charlson comorbidity score17. Clinical cure was defined as an absence of infection signs and fever registered in electronic history records. Microbiological cure was defined as a negative culture of focus infection after CZA treatment completion. The safety was registered as any adverse event related with treatment achieved in electronic medical record. Adverse event degree was classified according to Common Terminology Criteria for Adverse Events (CTCAE) Version 5.018. The infection were classified attending to EMA approval19. Bacteremia and admission in ICU were registered as a poor prognostic factor20,21. Bacterial identification and enzyme associated with antimicrobial resistance were collected (OXA-48 carbapenemase, KPC and extended-spectrum beta-lactamase (ESBL)). CZA was administrated as a standard dose of 2.5 grams intravenously every 8 hours with dose adjustments based on EGFR calculated CKD-EPI. EMA recommendations of time treatment according to infection onset were considered18. Causes of ending treatment considered were: worsening of the infection with CZA treatment, CZA resistance by isolated bacteria, CZA treatment toxicity, exitus of the patient, completing days of CZA scheduled treatment and clinical and/or microbiological. CZA combination therapy was defined as the receipt of Gram-negative targeted antibiotic for ≥48 hours with CZA.
Statistics analysis
Baseline characteristics of the patients were evaluated using descriptive statistics (proportion of patients, means, standard deviation (SD), medians, interquartile range (IQR)). In the analysis of subgroup, Chi-squared test was performed to identify the association between effectiveness and infection recurrence evaluated at 90 days with ICU admission, bacteremia and concomitant antibiotic therapy. T-student test was applied to cooperate effectiveness and Charlson index. Results were considered statistically significant if the p-value was less than 0.05. All analyses were calculated using SPSS Version 22.0 software.
RESULTS
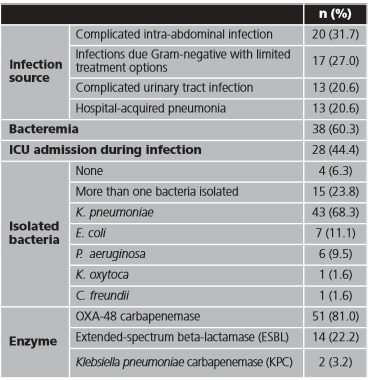
Between January 2016 and October 2018, 63 inpatients were treated with CZA; all of them were included in the study. The mean (SD) age was 64.3 (15.8) years and 39 (61.9 %) patients were male. Sixteen (25.4%) patients were admitted in Hematology, 11 (17.35%) in Internal Medicine, and 7 (11.1%) in Digestive Surgery (distribution by admitted unit are shown in table 1A of Appendix). In 20 (31.7%) patients, CZA was administrated as expanded use, before CZA was commercialized. Charlson Index mean (SD) was 4.27 (3.0). Infection characteristics are presented in table 1.
Previously to starting treatment, CZA susceptibility was verified in 56 (88.9%) patients.
Mean (SD) time until start of CZA treatment was 9.7 (10.7) days, and mean treatment days was 13.2 (10.5). In 19 (30.1%) patients, recommendations of time treatment by infection onset were exceeded, with an average of 4.5 (16.6) days. Treatment characteristics are shown in table 2.
Effectiveness
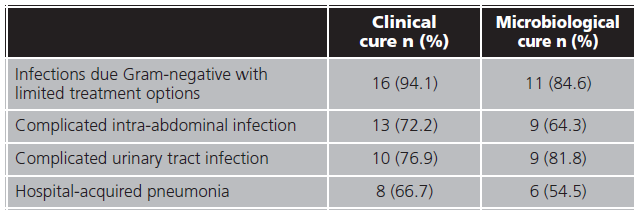
Microbiological cure were reached in 35 (55.6%) and were not obtained in 14 (22.2%) patients. A bacterial culture of infection focus for microbiological cure confirmation were not performed in 14 (22.2 %) patients. Forty-seven (74.6%) patients achieved infection clinical resolution and in 13 (20.6%) clinical cure were not evaluated. Clinical and microbiological cure proportion by infection source are shown in table 3.
Infection recurrence evaluated at 90 was registered in 23 (36.5%) patients, average age 60.0 (16.0) years, and 12 (52.2%) received CZA retreatment. The most isolated bacteria was K. pneumoniae, in 15 (65.2%) patients, and OXA-48 was the most common enzyme 15 (78.3%). The mean duration of CZA treatment were 10 days and in 9 (75.0%) patients concomitant systemic antibiotic were used. None of the recurrences developed CZA resistance and all of them were resolved.
Safety
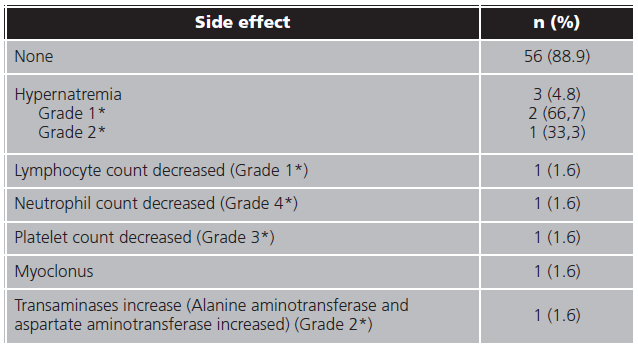
At least one side effect were registered in 7 (11.1%) patients and only one patient underwent two side effects. In 3 (4.8%) patients the treatment were interrupted because of the adverse events. In table 4 are resumed side effects frequency.
Subgroups analysis
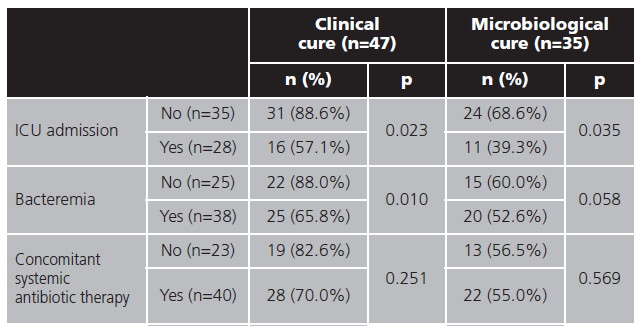
Differences in effectiveness (clinical and microbiological cure) was analyzed by Pearson's chi-squared test in difference subgroups shown in table 5.
Infection recurrence evaluated at 90 days, was higher in those with bacteremia (18 (60.0%) vs. 5 (20.0%) patients, p=0.003). No relation were found between ICU admission and infection recurrence during 90 days (12 (54.5%) vs. 11 (33.3%), p=0.118).
Median Charlson comorbidity Index was analyzed by Student's t-test in patients who reached clinical cure (3.9 (2.9) vs. 5.38 (3.0), p=0.122) and microbiological cure (4.3 (3.1) vs. 4.8 (3.0), p=0.654).
DISCUSSION
The upward incidence of nosocomial infections by Enterobacteriaceae pathogens such as Klebsiella pneumoniae, Escherichia spp. or Pseudomonas spp. is a health problem as WHO addressed in the Global Action Plan on Antimicrobial Resistance, published in 20152. Most of these pathogens has a broad spectrum of antimicrobial resistance based on carbapenemases and beta-lactamases enzymes. These factors complicate the treatment, increase treatment failure and mortality of nosocomial infections4,20. The current strategy is to find new antibiotics and antibiotic-inhibitors combinations that are able to escape these resistance mechanisms. Therefore, we followed out this evaluation of effectiveness and safety of CZA, a novel antibiotic-inhibitor, in clinical practice.
In our cohort, the most frequent source of infection was intra-abdominal (31.7%) followed by urinary and pulmonary, similar to Sousa et al.6 and Shields et al.23,24, and in contrast with other studies where pulmonary source was the most common21,22. K pneumoniae was the most CRE isolated (43%) followed by E coli (11.1%) and P aeruginosa (9.5%). These results contrast with the literature, but in our case proportion of K pneumoniae is lower6,21,22. In nearly a quarter of patients (23.8%) more than one CRE were isolated, which may have complicated patient's prognosis. As in Aleaddadi et al.22, the predominant carbapenemase was OXA-48 (81.0%), nevertheless in other cohorts KPC was the majority carbapenemase23,24. Bacteremia was confirmed in more than half of patients (60.3%), slightly higher than similar studies6. On the other hand, our proportion of ICU admission (44.4%) was lower than other cohorts21. It could be due to in our study, there were a high proportion of patients admitted in hematology service (25.4%), where immunocompromised patients are more common.
Regard to treatment characteristics, previous treatment failure was the majority beginning cause, and mean time from onset infection until start of CZA was greater than literature6. It could be due to three factors: 1) in almost a third of patients CZA was administrated as expanded use when treatment had to be approved by the hospital management and regulatory agencies; 2) subsequently, CZA was not included in pharmacotherapeutic guide, and it required management approval as well; and 3) when finally CZA was included in pharmacotherapeutic guide, it needs to be prescribed by Internal Medicine Service (with the exception of ICU patients), because a restrictive use of antibiotic in our hospital.
Concomitant systemic antibiotic therapy was registered in 63.5% of the patients, higher proportion than in other cohorts; aminoglycosides was the most combined antibiotic6,21.
A very low proportion of mortality at 14 and 30 days of treatment was observed (9.5% and 6.3% respectively), similar to Torres et al.8 (mortality at 30 days = 8.1%), Sternbach et al.10 (mortality at 30 days = 5.9%) or van Duin et al.25 (mortality at 30 days = 9.0%). However, mortality was lower than other series where 30-day mortality reached 25-50%22,26,27. It could be due to higher bacteremia frequency and Charlson Index in Alraddadi et al.22 study, due to more bacteremia presence in Caston et al.26, comparing with our population, and due to the higher number of patients in critical condition in King et al.27.
In our study, a high proportion of clinical cure (74.6%) was reached, similar to reported in literature, ranging between 68.8-91,9%6,8,21,22,26,28-32. Almost half of the patients reached microbiological cure (55.6%), low proportion comparing with literature, where it varies between 65.0- 91.2%6,10,29,30,32. Difference could be due to the fact that microbiological cure was not evaluated in 22.2% of our patients. Infections due Gram-negative with limited treatment options reached the highest proportions of clinical and microbiological cure, followed by cUTI, in contrast with other studies where HAP reached more proportion of infection resolution22. In a systematic review published by Zhong et al., clinical and microbiological response was superior in patients with cUTI, what could be explained by the renal excretion of avibactam28.
Recurrence of infection evaluated at 90 days (36.5%), was slightly higher than Sousa et al.6 and Alraddadi et al.22 studies (10.0% and 20.0% of recurrence at 90 days, respectively). None CZA resistance was achieved, even in infection recurrence, similar to low rates reported in literature, varying from 0-2.0%6,8,9,10,29.
Adverse event requiring discontinuation were only necessary in 3 (4.8%) patients (due to hypernatremia, neutropenia and transaminase increase). In similar observational studies, none discontinuations were achieved6,26. Even then no adverse event were registered in 88.9% patients and low proportion adverse events registered in our cohort were similar to others studies6,9,12,26,28.
Effectiveness subgroup analysis showed ICU admission as poor prognostic factor for clinical and microbiological cure, with differences statistics significant. This could be due to the fact that the pharmacokinetics in critical patients of ceftazidime is well known, however more studies are required for avibactam29.
In the case of bacteremia, results are similar, but statistical significance were not reaching in microbiological cure, due to patients in whom microbiological cure were not registered.
When it comes to concomitant antibiotic treatment, no differences were found in effectiveness (clinical or microbiological cure), similar to found in Jorgense et al.21 study.
In our cohort, bacteremia demonstrated the highest impact in recurrence infection evaluated at 90 days. Bacteraemia can cause infection in a remote focus that may not be detected in blood cultures and promote the premature end of CZA treatment.
Our study has several limitations. First one, missing data due to retrospective design of the analysis could distort the results, for example, the loss of almost a quarter of microbiological resolution data. On the other hand, reasons of exceeding recommendations of time treatment were not analyzed. Finally, a small sample size could influence in statically power of the study.
In conclusion, CZA has demonstrated efficacy and safety in clinical trials, but data in regular clinical practice may be differ from them. The effectiveness, evaluated as clinical and microbiological cure, were similar to others real clinical practice studies, and could confirm CZA as election therapy in CRE infection with a good safety profile and a low discontinuation proportion due to adverse event. Some factors, as the ICU admission and bacteremia were related with less effectiveness and more 90 days infection recurrence. An early CRE identification was essential for reached infection resolution, especially in patients with those poor prognostic factors.