INTRODUCCIÓN
Cancer is the second leading cause of death which causes a significant and increasing health burden for the Health Systems1.
In 2015, the use of antineoplastic drugs in Spain accounted for around 1,700 million euros per year, which represented a 35% of the direct costs of cancer and accounted for 10.04% of pharmaceutical expenditure2.
Prior to be released to the market, cancer drugs need to be evaluated in Oncology Clinical Trials (OCT). They are essential to evaluate the efficacy and safety of the new treatments. These drugs are often tested compared or combined with the standard protocol used in clinical practice. According to the Spanish legislation3, when an investigational drug is tested compared or combined with a standard of care in a sponsored clinical trial, standard pharmacotherapy and investigational drugs must be provided or refunded by the pharmaceutical company sponsoring the clinical trial. Even the drugs used in the control arm have to provide for free, without economic impact for the Health System. Therefore, we can define the cost avoided as the expense that would have had to be paid if the patient had not participated in an OCT4-5.
In the current scenario of cost containment, looking for saving strategies is needed in order to reduce the economic burden for the Public Health System. Oncologic patient's enrollment in clinical trials in which treatments prescribed are provided free of charge by pharmaceutical companies is an important source of savings for health institutions involved in cancer care.
In addition, in recent years there has been a growing number of OCT carried out in Spain according to available data of the Spanish Agency for Medicines and Health Products6. This means that the use of investigational drugs increasingly benefits a larger number of patients.
Even though, there is evidence of the intangible benefits of the OCT for patients, researchers and managers, but the tangible economic benefits are poorly documented7. Over the OCT period in which a patient participates in an OCT, all drugs being used within the OCT framework are being provided by the OCT s sponsor without any costs to the hospital. The objective of this study was to evaluate the economic effect of OCT, in terms of avoided costs in drugs, as a consequence of patient s enrollment. We calculated direct costs savings generated by the use of drugs provided by pharmaceutical companies while a patient participates in an oncology clinical trial.
METHODS
This is a retrospective observational study in a university hospital of the Public Health System of Madrid (Spain). Patients participating in active sponsored OCT in the hospital in April 2018 were included. To analyze savings, we calculated costs for each patient as if they had been treated with a standard protocol in clinical practice, from the inclusion of the patient in the study until April 2018, when the cross-section was carried out.
Data sources were: The software for investigational drugs management available in the Clinical Trials Unit in the Pharmacy Department, the Computerized Physician Order Entry (CPOE) program for oncologic pharmacotherapies prescriptions FarmisOncofarm®, the Electronic Clinical Record of each patient, the application Farmatools® for the integral management of the Hospital Pharmacy Service and Nomenclator database to consult official prices of medicines in Spain.
The following outcomes were measured: trial phase, type of cancer in study, investigational drugs, number of courses administered from the inclusion in the clinical trial, cost of treatment in the experimental arm and cost of treatment in the control arm. Patient s characteristics were also analyzed (gender, age, weigh and body surface area).
We calculated costs savings as follows:
It was calculated based on the Laboratory Sale Price inSpain of each involved drugs at the time of this study was carried out. If there were any discounts obtained by commercial agreements for any drug, they were not taken into account for the calculation of costs.
Calculation of the cost per milligram considering thatleftovers of the used vials are used for other elaborations.
We considered as standard pharmacotherapy the treatment schedule approved by the Pharmacy and Therapeutics Commission for each type of cancer.
Duration: time that the patient has been receiving treatment within the OCT framework.
Number of courses received while participating in an OCT. If the scheme of the standard therapy has a management pattern different from that of the experimental arm the number of corresponding cycles for the same period of time was taken into account.
Weight, size and body surface has been consulted forthe calculation of individualized doses.
In those patients who had not yet completed the treatment within the OCT at the date of the cross-section, only the cost of the medication dispensed until then was considered.
Other direct expenses were not taken into account, such as the cost of the consumable material used in the preparation and administration of the drugs, the costs of the diagnostic or imaging tests, hospital stays and staff costs were not taken into account. Moreover, costs of concomitant treatments were not quantified (premedication of chemotherapy, colony stimulating factors, antiemetics, systemic corticosteroids, etc.).
RESULTS
A total of 50 OCT involving 155 patients were analyzed. On average age of participants was 61.68 years (20-88 years) and the male/female ratio was 0.74.
Regarding investigational drugs in OCT analyzed, there were a total of 39 drugs involved (table 1). Six of them (15.4%) were chemotherapies, and 33 (84.6%) were biologic drugs (32 antibodies among those who are 6 lymphocite T checkpoint inhibitors; and 1 oncolytic virus).
Regarding administration routes of these drugs, 21 (53.8%) are administered orally, 16 (41%), intravenously and 2 (5.1%) intramuscularly.
In many cases, these investigational drugs were already approved and used in clinical practice for other indications or other types of cancer. We detected that 14 (35.9%) of these involved drugs were available in our center for other uses than those studied in these trials.
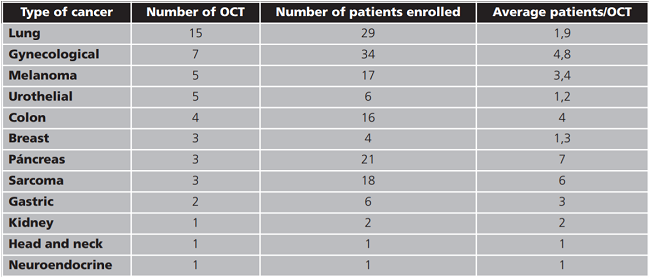
The type of cancer most frequently studied in the OCT evaluated was lung cancer. The number of OCT and enrolled patients according to the type of cancer are shown in table 2.
Table 2. Clinical trials in oncology and number of patients enrolled according to the type of cancer.
Involved patients remained in the clinical trials for 10.6 months on average.
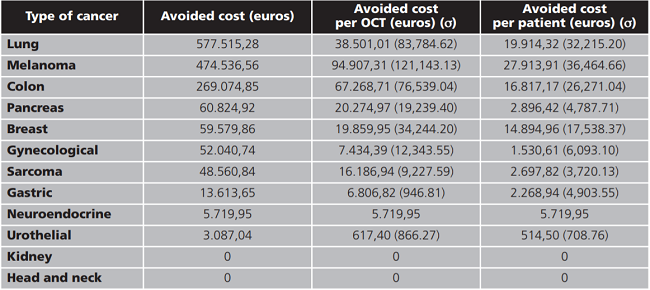
Total avoided cost in the 50 OCT analyzed was 1,564,943.59 euros. On average 31,298.87 euros per OCT and 10,096.41 euros per patient were saved in our center. Savings according to the location of the cancer are shown in table 3.
Taking into account the average savings per patient observed in our study and the number of patients enrolled in OCT, the estimated global avoided costs in 2018 in our hospital was 1,417,739 euros. Table 4 shows the breakdown of this estimation according to the type of cancer.
DISCUSSION
Patients enrolment in OCT leads to important cost savings in oncologic treatments. Researches in which medications are provided or refunded by pharmaceutical companies sponsoring them reduces the high economic burden of cancer patients care. This study proves that OCT contributes not only to scientific progress, but also helps to the sustainability of the Public Health System8.
In recent years, several studies analyzing costs avoided in oncological medicines related to patient s enrollment in clinical trials have been published. Mañes-Sevilla et al.8 in a retrospective study where 37 clinical trials researching drugs for breast cancer in Spain observed that 957,246 euros were saved in three years in which 89 patients were included in OCT. This amount is lower than that obtained in our study, probably because they included only clinical trials in breast cancer, whose patients generate a lower avoided cost than that obtained in other types of cancer.
Uecke et al.9 quantified the avoided costs in medicines in 11 hospitals in Germany over three years. They analyzed 88 clinical trials with oncological drugs in which they found costs savings of 1.5 million euros. Calvin-Lamas et al.10 evaluated the pharmaceutical expenditure savings in prostate cancer medicines as a consequence of patient's enrollment in clinical investigation. In 18 OCT observed avoided cost of 696,002 euros during a study period of 18 years.
We observed that, in one year, almost one and a half million euros, around ten thousand euros per patient, were saved because of patient s participation in OCTs in a single hospital. These amounts are consistent with the other studies mentioned above.
Savings in medications associated with clinical trials are expected to increasingly grow in coming years since there are an incremental trend in the number of clinical trials authorized in Spain, mainly in the field of oncology according to data from the Spanish Agency of Medicines6.
As far as we know, published studies are focused in one type of cancer, but there are not available data evaluating costs savings associated with clinical trials where savings comparing different types of cancer in OCTs in the same study. When comparing researches on pharmacotherapies of different cancers, we detected that lung cancer and melanoma clinical trials led to the highest avoiding costs in our center, probably because in both pathologies, the immunotheraphie and oral antineoplastics, which are more expensive drugs, are frequently used as standard treatment in clinical practice.
Other expenses derived from the participation of patients in OCT have not been taken into account, such as the costs of image and analytical diagnostic tests, personnel costs, hospital stays, associated concomitant medication (antiemetics , antidiarrheals, systemic corticosteroids, colony stimulating factors), although several studies have been published in which it is concluded that these expenses are very similar in patients receiving investigational treatment and those receiving the standard therapy11-13.
There are some limitations of this study. The number of courses corresponding to the same period of time that the patient was receiving treatment within the clinical trial was taken into account, but in most cases we do not know if the patient had reached the same survival and therefore the same duration of treatment in the event of not having participated in that OCT and having been treated with standard therapy.
In order to get external validity of our results, avoided costs were calculated based on official cost in Spain of involved medicines. This way we could compare our results with other hospitals in Spain. However, because of this, savings might be slightly overestimated if there were any discounts offered by the pharmaceutical companies since they were not taken into account.
Nevertheless, it has to be said as well that, OCTs in some cases, could induce prescriptions of new cancer drugs previously investigated in those OCTs when released. When investigational drugs have proved efficacy and safety patients are preferred to continue with the initiated treatments in OCTs. But, once finished the research, these medications are not provided free of charge anymore which increases expenses in cancer drugs after participating in an OCT.
CONCLUSION
It s known that patient s enrollment in OCTs offer the possibility of accessing therapies not yet available, and being able to contribute to scientific progress. In addition to this, our study proves that OCTs can contribute toward a sustainable Public Health System regarding oncologic patient's pharmacotherapies which are associated with the highest economic impact of cancer care.