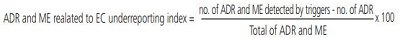INTRODUCTION
The manipulation of industrialized pharmaceutical forms is often necessary in hospitals to ensure that inpatients receive adequate treatment according to their pathophysiological needs. In addition, this procedure needs to be safe and effective, not exposing the patients to an unnecessary risk1. Extemporaneous compounding (EC) aims to meet this need and can be understood as preparing a drug for a specific individual in response to an identified need, usually performed for pediatric and geriatric patients, patients with invasive devices such as nasogastric tube, patients with dementia and rare diseases2,3. EC occurs when there is no other option available on the market, when the product available is inappropriate4 or when it is necessary to improve patient adherence and effectiveness of a drug, especially in patients with dysphagia and neurodegenerative diseases5.
EC is common worldwide. In an Ireland hospital elderly care unit, about 35.1% of patients received at least one EC medication, which were most commonly modified to facilitate fractional dosing. Moreover, of the 44 unlicensed modifications, 14 were evidence-based and 30 were not6. According to Fodil et al. (2017),7 40.3% of geriatric patients receive EC medication in long-term care units in teaching hospitals in Paris, France. In 104 cases, at least one medication could not be safely modified, including 26 cases in which none of the prescribed drugs were safe to crush or open. Recent data shows that three out of four patients hospitalized in a Brazilian hospital receive at least one medication that needed EC8. EC was identified in 88 different drugs of the 253 standardized drugs in the hospital pharmacy. However, there are 29 pharmaceutical alternatives in the Brazilian market for the 88 modified drugs, possibly decreasing EC by 28.5%8. This process can affect the pharmaceutical characteristics of the product and its therapeutic result, increasing the possibility of occurring adverse drug reaction (ADR), specially for drugs with a narrow therapeutic index, cytotoxic, teratogenic, hormones, steroids and for drugs that may irritate the gastrointestinal tract9.
Adverse drug events (ADE) can be defined as any harm caused to patients arising from drug use, such as medication errors (ME), ADR, allergic reactions, and overdoses10. EC can generate ME, since approximately 60% of the medications are not commercially available in the concentration required by the patient and there is no standardization of the final concentrations in extemporaneous preparations11. In this context, the aim of this study was to assess the causality between ADR and EC and to analyze ME related to the EC using the spontaneous reports generated by the healthy team in a medium sized general hospital.
MATERIAL AND METHODS
Ethical approval
The study was approved by Américo Brasiliense State Hospital Research Ethics Committee and by the School of Pharmaceutical Sciences, São Paulo State University (UNESP) Ethics Committee (CEP/FCFAR number: 05752818.1.0000.5426).
Study design
An observational cross-sectional study was performed in a medium-complexity public hospital, based on the guideline Strengthening the Reporting of Observational Studies in Epidemiology (STROBE)12. The institution has an electronic health record, which includes, patient's medical history, diagnoses, medications, treatment plans, allergies, radiology images and laboratory test results. All health professionals register their assessments, such as physicians, psychologists, physiotherapists, speech-language pathologists, pharmacists, nurses, nursing technicians and social workers.
In a cross-sectional study, the researcher measures the outcome and the exposures in the study participants at the same time and the participants are selected based on the inclusion and exclusion criteria set for the study. Cross-sectional studies are faster and are inexpensive, when compared with other studies like cohort. These types of study give information about the prevalence of outcomes or exposures, which can be useful for designing the cohort study. In contrast, since this is a one-time measurement, it is difficult to originate causal relationships. The prevalence of an outcome depends on the incidence of the disease as well as the length of survival following the outcome13.
Participants and data collection
ADR and ME spontaneous reports registered between August 2017 and July 2018 were collected, covering 53 beds in all hospital's wards. Inclusion criteria for causality assessment comprised all reports with any reference to an EC performed for patients in the hospital wards. The exclusion criteria included reports with no reference to EC, lack in data necessary for causality assessment and those that came from other sections and services of the hospital, since no patients would be hospitalized there. After that, it was performed a retrospective search for ADR, ME and changes in biochemical and hematological profile in the electronic health record. Finally, it was conducted the causality assessment between the ADR and the EC and the analysis of ME.
Data analysis
After data collection, the causality between the triggered ADR and the EC was performed using the World Health Organization – Uppsala Monitoring Center (WHO-UMC) algorithm since it is more suitable for monitoring ADR in a hospital environment14. The algorithm includes the categories “certain”, “probable/likely”, “possible”, “unlikely”, “conditional/unclassified” and “unassessable/unclassifiable”15. The causality assessment of ADR was performed by using clinical judgment, that considered the temporal relationship between the occurrence of the event and drug use; ADRs previously described in medication package insert; pharmacologic plausibility, whether the mechanism of action of the drug may produce the event and exclusion of confounding variables that may explain the case, such as clinical condition of the patient and other drug-related problems.
The analyses of potential ME related to the EC were performed by the National Council for Coordination of Reporting and Prevention of Medication Errors (NCCMERP) algorithm, which includes stratified categories: “no error: A”, “error, no harm: B, C and D”, “error, harm: E, F, G and H” and “error, death: I”16. The following information was used in both analysis: date of the incident, description of the report, mentioned medication, incident identified in the medical record, type of diet that the patient was receiving, patient's sex, height, weight, age and body mass index, period of hospitalization, pharmacotherapy, biochemical and hematological profile. Extemporaneous compounding techniques were classified in tablet dispersion and splitted tablet17.
Furthermore, studies comparing the pharmacokinetics between the administration of the intact tablet and the extemporaneously prepared drug or splitted tablet as well as drug polymorphism in solution were searched on PubMed to complement the analysis. The MeSH terms searched were: [(oral solution) AND tablet) AND “name of the drug”]; [(Nasogastric) AND “name of the drug”; (Bioavailability) AND “name of the drug”]; [(Absorption) AND “name of the drug”]; [(Splitting) AND “name of the drug”]; [(Pharmacokinetics) AND “name of the drug”]; [(Crushed) AND “name of the drug”]; [(Solid-state) AND “name of the drug”]; [(Crystalline structure) AND “name of the drug”]; [Crystalline) AND “name of the drug”]; [(Polymorph) AND “name of the drug”]. The medication package insert were also used to search for ADR.
The following equations were used to estimate the incidence and the underreporting of ADR and ME related to EC, respectively:
RESULTS AND DISCUSSION
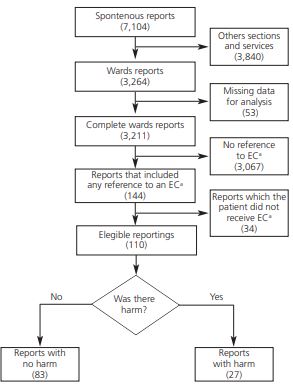
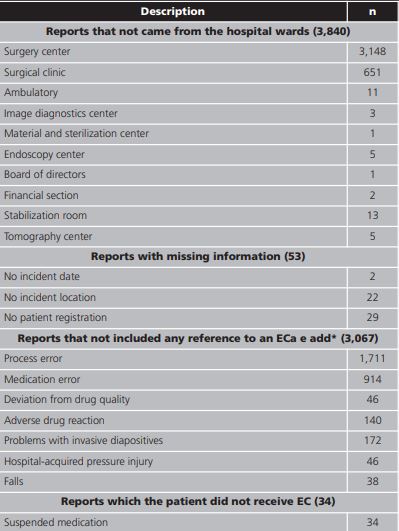
During the period, the hospital team generated 7,104 spontaneous reporting, of which only 144 were related to EC (Figure 1; Table 1). Thirty-four reports were excluded because the patient did not receive the EC preparation due to clinical pharmacist action suggesting pharmaceutical alternatives or changes in the administration route (Table 1). Then, from the 110 reports that mention EC analyzed, 85 described the tablet dispersion technique and 25 described splitted tablet technique.
The EM and ADR causality assessment showed that 83 reports resulted in no harm to patients whereas 27 resulted in harm (Figure 1). From these 27 reports were possible to classify 3 ADR as “probable” and 23 ADR as “possible” related to the EC whereas 4 EM were classified as “error, harm: category E” (Supplementary file).
Only 4.5% (144/3,211) of the reports mentioned EC. ADR and ME possibly associated to EC was estimated in 24% (27/110) of the reports. Furthermore, only 4 EM were described in the analyzed reports giving an underreporting index of 0.87 (26/30).
Little is known about the relation between ADR, ME and EC even though this procedure is quite often in hospitals. The ADR causality assessment and the EM analysis showed relationship to EC of some drugs leading to harm to the patient such as changes in patient's biochemical and hematological changes, mental confusion, agitation, constipation and vomiting. Few studies highlight the risk of EC. In one of them18, the EC practice had negative effects on medication use, causing minor to severe harm such as diarrhea, stomach perforation and can lead the patient to death. Other authors19 reported the death of a patient after daily administration of a crushed prolonged-release nifedipine tablet by nasogastric tube, concluding that procedure caused severe hypotension due the high plasma concentration of nifedipine generated by the immediate release of the entire 90 mg dose. The EC of enteric coated drugs are specially linked to the incidence and severity of ADR in upper and lower gastrointestinal tract20. Yet, conventionalcoated drugs can also cause ADR due to EC, since this process can increase the speed of absorption and bioavailability21.
The rate of spontaneous reporting by health professionals is low worldwide and is not a mandatory requirement in most countries22. In Brazil, according to Varallo et al. (2018)23, the perception and the cause of underreporting by the health team are indifference, distrust, ignorance and guilt. In addition, professionals believe that only the nursing team must report. This present study evidence the underreporting of ADR and ME possibly related to EC since only 4 ME were reported. Only 4.5% of the reports mentioned EC, whereas ADR and ME possibly associated to EC was estimated in 24% of the reports.
Studies about EC pharmacokinetics/pharmacodynamics and drug polymorphism in solution are scarce yet essential to complement the causal assessment and to discriminate if the ADR and ME came from the EC procedure or the drug itself. A clinical study demonstrated that the preparation of amlodipine in suspension and the intact tablet are bioequivalent, despite the need to observe EC stability24. However, another clinical study showed lack of bioequivalence between preparing a clopidogrel solution and the intact tablet. The patients that received the EC preparation showed an increase in the absorption speed in 40 minutes, and an almost two-fold increase in the area under the curve21. Nonetheless, EC can also promote adherence and can improve the pharmacotherapeutic effectiveness in elderly patients with dysphagia and neurodegenerative diseases, as reported by Mastroianni and Forgerini (2018).
EC are indispensable to treat a range of conditions and it occurs in health services worldwide. However, there seems to be a lack of knowledge about EC safety. Data of this study shows that only a small number of reports includes the EC. However, one out of four reports that mentioned EC leads to patient harm. The hospital's guideline for EC practice allows the procedure under some circumstances, such as the unavailability of the active pharmaceutical ingredient in the market or a basic dosage form absent in the concentration compatible with the needs of the patient17.
The professional continuing education as well as periodic training seems to develop abilities and skills to ensure the quality of EC preparation, to improve the professionals' perception of this practice and the culture of spontaneous reporting23. Additionally, Glass and Haywood (2006)25 and others26 provide a management guideline for EC practice. In the absence of the proper pharmaceutical form, a therapeutic alternative should be dispensed. If none is available, a pharmacopeia formula should be dispensed. The third option would be a research for stability-validated formulation. The final decision relays on the use of scientific principles and tablet dispersion.
This study has some limitations. Data collection was conducted in a general medium-complexity public hospital. Hence, data may not be generalizable to other types of institutions. The causality assessments were conducted using medical record from patients that had already been discharged. This approach could delay the identification of potential confounding variables that were not described in the medical records. The algorithms used were not developed to evaluate the use of EC. To reduce the error of analysis, it was necessary to complement it with studies of pharmacokinetics and polymorphism. Many inpatients that necessitated EC had poor health conditions or several comorbidities and polypharmacy. These factors might confound causality assessment for ADR. Further studies are necessary to evaluate the efficacy and safety of EC. Future studies may investigate the use of EC as a trigger tool for ADR since this study shows that one out of four reports that mentioned EC leads to patient harm.
CONCLUSION
Extemporaneous compounding are rarely mentioned in spontaneous reports (4.5%). One out of every four reports that mentioned EC is linked to ADR and ME (24%). Furthermore, there are an underreporting index of 0.87 of ADR and ME related to EC. That is, among 10 events triggered, 8 were underreported. This is, to the best of our knowledge, the first study that associate the ADR and ME to EC, a very common practice in hospital pharmacy. Considering that EC are indispensable to meet inpatients’ pharmacotherapeutic needs, the data suggest triggering EC to identify underreporting of ADR and EM.