Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Pharmacy Practice (Granada)
versão On-line ISSN 1886-3655versão impressa ISSN 1885-642X
Pharmacy Pract (Granada) vol.4 no.2 Redondela Abr./Jun. 2006
|
Original research |
Clinical practice and costs of treating catheter-related infections with teicoplanin or vancomycin
Práctica clínica y costes al tratar infecciones por catéter con teicoplanina o vancomicina
Steven SIMOENS, Nik DE CORTE, Gert LAEKEMAN.
|
ABSTRACT Objectives: To elicit actual clinical practice of treating intensive
care unit patients with catheter-related infections with teicoplanin or
vancomycin from a hospital perspective. As clinical trials have demonstrated
similar efficacy of these glycopeptides, a cost-minimisation analysis
was also carried out. Key words: Glycopeptide. Teicoplanin. Vancomycin. Cost minimisation analysis. Belgium. |
RESUMEN Objetivo: averiguar la práctica clínica real del tratamiento
en las unidades de cuidados intensivos sobre las infecciones relacionadas
con catéteres con teicoplanina o vancomicina desde la perspectiva
de un hospital. Como los ensayos clínicos han demostrado que la
eficacia de estos glucopéptidos es simular, también se realizó
un análisis de minimización de costes. Palabras clave: Glucopéptidos. Teicoplanina. Vancomicina. Análisis de minimización de costes. Bélgica. |
Steven SIMOENS. MSc PhD, Professor of Pharmaco-economics. Research Centre for
Pharmaceutical Care and Pharmaco-economics, Katholieke Universiteit Leuven,
Belgium
Nik DE CORTE. PharmD, Health Economics, Pricing and Reimbursement Manager. Sanofi-Aventis,
Brussels, Belgium.
Gert LAEKEMAN. PharmD PhD, Professor of Pharmaco-therapeutics. Research Centre
for Pharmaceutical Care and Pharmaco-economics, Katholieke Universiteit Leuven,
Belgium .
INTRODUCTION
Glycopeptides are used in the treatment of infections instigated by Gram-positive bacteria, particularly staphylococci, enterococci and pneumococci. This family of antibiotics is increasingly used as a result of the rising number of infections caused by organisms that are resistant to penicillin and methicillin. The two glycopeptides licensed for use in Belgium are teicoplanin and vancomycin. Comparative trials of teicoplanin and vancomycin have reported no significant differences in their efficacy, a common finding in the field of antibiotics.1,2 However, potential differences in treatment costs may play a role in the choice between these two glycopeptides. A cost comparison not only needs to take into account the higher drug acquisition costs of teicoplanin, but also explore the cost implications of any differences in delivery and monitoring of these two glycopeptides.
To date, there is little evidence of how teicoplanin and vancomycin are used in actual clinical practice and of the different cost drivers associated with their use (drug acquisition costs, costs of materials and nursing time required for drug preparation and administration, and costs of laboratory tests). Two studies have analysed patient records to determine resource utilisation and costs associated with treatment with teicoplanin and vancomycin.3,4 Although such an approach has the benefit of being precise and comprehensive, it is not straightforward to link resource utilisation information contained in patient records to the drug and type of infection under study when patients receive several drugs to treat multiple comorbidities. Moreover, analysing patient records is time- and resource-intensive. An alternative approach is to gather the opinion of practicing physicians to shed light on actual clinical practice and identify the various cost drivers associated with the use of teicoplanin and vancomycin.
The aim of this study is to carry out an economic evaluation of the use of teicoplanin and vancomycin in the treatment of intensive care unit patients with catheter-related infections in Belgium from a hospital perspective. In light of the similar efficacy of teicoplanin and vancomycin, the evaluation takes the form of a cost-minimisation analysis. In a first instance, information about the resource utilisation profile associated with teicoplanin and vancomycin is gathered from a panel of intensive care unit physicians. This allows us to elicit actual clinical practice in terms of dosing schedule, route of administration, and utilisation of laboratory tests with teicoplanin and vancomycin. In a second instance, resource utilisation is valued at unit costs pertaining to University Hospitals Leuven to identify which one of these two glycopeptides incurs the lowest treatment cost. The results of this study will be useful to intensive care unit physicians and hospital pharmacists wishing to gain additional insight into clinical practice and costs of treatment with teicoplanin and vancomycin.
METHODS
A Delphi survey technique is used to map resource utilisation associated with teicoplanin and vancomycin in a hospital setting. This technique is frequently employed to correct for insufficient data by harnessing the experience of an expert panel.5 It is a suitable instrument for eliciting resource utilisation associated with teicoplanin and vancomycin given the lack of knowledge of actual clinical practice and lack of data on the various cost drivers associated with the use of these glycopeptides.
The Delphi technique seeks to attain consensus of opinion of an expert panel through consecutive rounds of structured questionnaires, interspersed by controlled feedback to participants. For the purposes of this study, nine intensive care unit physicians were selected to participate in the successive rounds of questionnaires. Only physicians who had extensive knowledge and clinical experience with using teicoplanin and vancomycin were enrolled. Physicians worked in general and university hospitals, geographically spread out across Belgium, in order to ensure representativeness of the sample and generalisability of results. An information letter was sent to physicians setting out the aims of the study and explaining the nature of their contribution as this has been shown to improve the validity of the Delphi technique.6 Physicians received a fee for participation in the study.
The aim of the first round was to solicit the opinion of each individual physician with respect to resource utilisation associated with teicoplanin and vancomycin in treating intensive care unit patients with catheter-related infections. The initial questionnaire was piloted and validated by three opinion leaders in this area of clinical practice. Face-to-face interviews were employed in the first round because this has been found to increase response rates in successive rounds.7 The questionnaire contained a mix of open-ended and closed questions about dosage of teicoplanin and vancomycin, duration of treatment with glycopeptides, material required to prepare and administer teicoplanin and vancomycin, and utilisation of laboratory tests (microbiology test and serum level monitoring to determine the appropriate dose and avoid toxic levels). Other tests such as haematology and biochemistry tests that are carried out daily on intensive care unit patients irrespective of the presence or absence of a catheter-related infection were not taken into account as these do not generate an incremental cost.
Adverse effects were not considered given that the adopted methodology is not suitable for eliciting differences in the frequency of adverse events associated with treatment with teicoplanin and vancomycin. Such information about the frequency of adverse events is better derived from randomised controlled trials or large observational databases. Moreover, previous evidence suggests that costs associated with treating adverse events are likely to be small given that nephrotoxicity and ototoxicity are relatively uncommon with teicoplanin and with vancomycin (except for patients with renal failure or patients who are given concomitant aminoglycosides).8
The findings of the initial questionnaire were summarised using descriptive statistics and fed back to participants through a second questionnaire. The second questionnaire was completed by means of a telephone interview. In this second round, each physician was confronted with the responses of all participants and was given an opportunity to modify his/her answers, taking into account the experience of colleagues. Although the number of rounds that can be undertaken is in principle not limited, evidence suggests that two rounds are sufficient to reach consensus of opinion of the expert panel.9
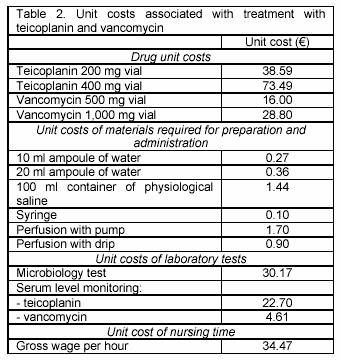
Data on the resource utilisation profile associated with teicoplanin and vancomycin were subsequently combined with information about unit costs pertaining to 2005 to generate cost estimates. Drug unit costs reflect official prices charged by pharmaceutical companies to hospitals and do not take into account possible discounts that hospitals may obtain. Other unit costs of materials, laboratory tests and nursing time pertain to University Hospitals Leuven, a 1,900-bed hospital where physicians and residents carry out more than 18,000 major procedures on inpatients per year. Unit costs of laboratory tests not only incorporate the acquisition cost, but also overheads of infrastructure, supervision and production. The unit cost of nursing time is based on the gross wage of a nurse with 12 years of experience working in the intensive care unit. All data were processed and analysed using Microsoft Excel.
RESULTS
Table 1 displays the resource utilisation profile associated with teicoplanin and vancomycin in the treatment of intensive care unit patients with catheter-related infections, based on consensus from the expert panel. Results of the first and second questionnaire were consistent, with only marginal changes related to administration of vancomycin being reported. The fact that consistency was reached after only two rounds of questionnaires informed the decision not to carry out any additional rounds.
With respect to the dosing schedule, a loading dose is required for teicoplanin. Although a loading dose of 400-800 mg/day is recommended, physicians tended to use a higher median dose of 1,200 mg/day in actual clinical practice. The maintenance dose was in line with the recommendations of the drug information leaflet, amounting to 400 mg/day for teicoplanin and 2,000 mg/day for vancomycin. Treatment duration of 12 days was similar with teicoplanin and vancomycin.
The majority of physicians (five out of nine) administered teicoplanin by intravenous injection. One physician preferred intravenous perfusion with pump and two physicians chose perfusion with drip. One physician opted for perfusion with pump half of the time and perfusion with drip the other half of the time. Although administration of teicoplanin by intramuscular injection is feasible, this was not done in actual clinical practice. Vancomycin was administered by perfusion with pump by all nine physicians. Five physicians administered vancomycin once daily, three physicians preferred a twice-daily schedule, and one physician administered every six hours. Real-life adherence of most physicians to a once-daily administration schedule can be questioned given that evidence of the effectiveness of vancomycin is mainly derived from trials using a multiple-daily administration schedule.1,2
The use of teicoplanin and vancomycin may be accompanied by the need to conduct laboratory tests. No differences were observed in the number of microbiology tests performed per week between teicoplanin and vancomycin. Only two out of nine physicians monitored serum concentrations during treatment with teicoplanin. This contrasts with treatment with vancomycin, during which all nine physicians monitored serum concentrations.
The resource utilisation profile associated with teicoplanin and vancomycin was valued at unit costs displayed in Table 2 to attain cost estimates. Mean costs of treating intensive care unit patients with catheter-related infections amounted to 1,272 with teicoplanin and 1,041 with vancomycin (see Table 3). Higher treatment costs with teicoplanin can be attributed to more elevated drug acquisition costs (1,076 versus 795 ) and higher costs of nursing time required for drug preparation and administration (42 versus 21 ). Given the similar treatment duration with teicoplanin and vancomycin, the more elevated drug acquisition costs with teicoplanin arose from its higher unit cost, which is more than double that of vancomycin (see Table 2). The practice of monitoring serum concentrations during treatment with vancomycin contributed to higher costs of laboratory tests with vancomycin (150 versus 217 ).
The disparity in treatment costs between teicoplanin and vancomycin originated from differences in drug acquisition costs and costs of laboratory tests. However, the magnitude of these cost drivers is likely to vary across hospitals. The extent to which unit costs would need to change in order for treatment costs with teicoplanin to be equal to that with vancomycin was investigated by means of a threshold analysis. For instance, hospitals may vary in their ability to negotiate discounts with respect to drug acquisition. If the unit cost of teicoplanin would fall by 21%, mean treatment costs per patient would be the same with teicoplanin and vancomycin. The unit cost of the serum level monitoring test is also likely to vary and depends, for instance, on the number of tests that are carried out at a hospital during a year.10 The lower unit cost of monitoring serum levels with vancomycin as compared with teicoplanin (4.61 versus 22.7 ) reflects the fact that University Hospitals Leuven predominantly treats intensive care unit patients with catheter-related infections with vancomycin. In hospitals that rely less on vancomycin, the unit cost of monitoring serum levels with vancomycin is likely to be higher. No difference in mean treatment costs per patient between teicoplanin and vancomycin would be observed if the unit cost of monitoring serum concentrations with vancomycin would increase from 4.61 to 23.20 and the unit cost with teicoplanin remained constant.
A sensitivity analysis was carried out to examine the impact on the findings of the uncertainty surrounding the time needed to prepare and administer teicoplanin and vancomycin. Preparation of teicoplanin may take more time given that rapid reconstitution leads to frothing. Therefore, our base-case analysis assumed that teicoplanin took seven minutes to prepare and administer as opposed to two minutes with vancomycin. A Scottish study, however, observed that administration of vancomycin was slower, leading to an identical nursing time required for preparation and administration of 12 minutes for teicoplanin and vancomycin.3 If these estimates would be used, mean treatment costs per patient would amount to 1,302 with teicoplanin as compared to 1,146 with vancomycin.
DISCUSSION
This study has contributed to eliciting actual clinical practice related to the use of teicoplanin and vancomycin in the treatment of intensive care unit patients with catheter-related infections. The findings indicate that physicians administer higher loading doses of teicoplanin than recommended in the drug information leaflet. Although teicoplanin can be administered by way of intramuscular injection, this does not seem to be routine practice. Even though vancomycin has a shorter half-life of 4-6 hours than teicoplanin (30 hours) and evidence of its effectiveness is mainly derived from trials using multiple-daily administration schedules, five out of nine physicians administered it on a once-daily basis. With respect to routine serum monitoring, this study found that physicians always monitored serum levels during treatment with vancomycin as opposed to its rare use during treatment with teicoplanin.
Given the similar efficacy of these two glycopeptides, a cost-minimisation analysis was also carried out. The findings showed that the more elevated treatment cost with teicoplanin mainly originates from higher drug acquisition costs. Differences in drug acquisition costs between teicoplanin and vancomycin are in part influenced by the steady decrease in the unit cost of vancomycin as a result of generic competition. More frequent monitoring of serum concentrations generated higher costs of laboratory tests during treatment with vancomycin. However, resource utilisation advantages from fewer laboratory tests associated with teicoplanin only partially offset higher drug acquisition costs. Treatment costs of teicoplanin and vancomycin turned out to be sensitive to changes in drug unit costs and unit costs of the serum level monitoring test. The findings of our study are similar to those of a Scottish and a Spanish study that determined treatment costs based on patient records.3,4 This indicates that the Delphi survey technique is an appropriate alternative method to analysing patient records when estimating resource utilization and costs in an economic evaluation.
The findings must be interpreted with the following caveats in mind. The Delphi panel consisted of nine experts who practiced in a variety of hospitals across Belgium, enhancing representativeness of study participants and generalisability of findings. Although the study enrolled a small number of physicians, other studies employing the Delphi technique have enrolled a similar number of participants.9 Information about some unit costs was derived from a 1,900-bed university hospital and may not be comparable to those pertaining to other hospitals. Potential variation in unit costs was taken into account by means of a threshold analysis which investigated how much unit costs would have to change for treatment costs with teicoplanin and vancomycin to be equal.
It should be noted that costs are one of the factors informing the choice of physicians between teicoplanin and vancomycin. Other factors that need to be taken into account include route of administration, patient profile and the occurrence of adverse events, such as nephrotoxicity, ototoxicity and Red man syndrome (i.e. erythema, pruritus and flushing of the upper torso). Teicoplanin offers the advantage over vancomycin that it has a longer half-life, making once-daily administration through different routes feasible. Nephrotoxicity, ototoxicity and Red man syndrome are relatively uncommon during treatment with teicoplanin, implying that serum monitoring is not necessary.1 On the other hand, physicians have greater clinical experience with vancomycin and incidence of ototoxicity is low. Nephrotoxicity during treatment with vancomycin has been reported, but only in patients with renal failure, pseudomembranous colitis or in patients who are given concomitant aminoglycosides.8 Monitoring of serum concentrations in these patients is recommended. Treatment with vancomycin is associated with the occurrence of Red man syndrome, although this can be avoided by slowing down the infusion rate or by prior administration of a histamine H1-receptor antagonist.11
CONCLUSIONS
This analysis of clinical practice regarding treatment of catheter-related infections with glycopeptides indicated that physicians tend to administer higher loading doses of teicoplanin than recommended in the drug information leaflet. Even though effectiveness data of vancomycin principally originate from trials using multiple-daily administration schedules, some physicians administer it on a once-daily basis. The analysis of costs showed that treatment with teicoplanin is more expensive than with vancomycin. This is because lower costs of laboratory tests with teicoplanin only partially offset higher drug acquisition costs. In addition to efficacy and costs, other factors such as route of administration, patient profile and adverse effects need to inform the choice between teicoplanin and vancomycin.
ACKNOWLEDGEMENTS
Financial support for this study was received from Sanofi-Aventis. The authors have no conflicts of interest that are directly relevant to the content of this manuscript. The authors would like to express their gratitude to Alexander Van Der Cruysse (Sanofi-Aventis) and to Nathalie Delesie, Annelies Peetermans, Sofie Schollaert, Mieke Suenens, Els Swiggers and Pieter Vermeersch who contributed to this study as part of their fourth-year pharmacy course on pharmaceutical care and policy.
|
References |
1. Wood MJ. The comparative efficacy and safety of teicoplanin and vancomycin. J Antimicrob Chemother 1996; 37: 209-22. [ Links ]
2. Zeckel ML. A closer look at vancomycin, teicoplanin, and antimicrobial resistance. J Chemother 1997; 9: 311-35. [ Links ]
3. Davey PG, South R, Malek MMH. Impact of glycopeptide therapy after hospital discharge on inpatient costs: a comparison of teicoplanin and vancomycin. J Antimicrob Chemother 1996; 37: 623-33. [ Links ]
4. Abad F, Calbo F, Zapater P, Rodriguez-Vilanova F, Garcia-Perez LE, Sacristan JA. Comparative pharmacoeconomic study of vancomycin and teicoplanin in intensive care patients. Int J Antimicrob Agents 2000; 15: 65-71. [ Links ]
5. Powell C. The Delphi technique: myths and realities. J Adv Nurs 2003; 41: 376-82. [ Links ]
6. Whitman N. The committee meeting alternative: using the Delphi technique. J Nurs Adm 1990; 20: 30-7. [ Links ]
7. McKenna HP. The Delphi technique: a worthwile approach for nursing? J Adv Nurs 1994; 19: 1221-5. [ Links ]
8. Wilson APR. Comparative safety of teicoplanin and vancomycin. Int J Antimicrob Agents 1998; 10: 143-52. [ Links ]
9. Hasson F, Keeney S, McKenna H. Research guidelines for the Delphi survey technique. J Adv Nurs 2000; 32: 1008-15. [ Links ]
10. Vacani PF, Malek MMH, Davey PG. Cost of gentamicin assays carried out by microbiology laboratories. J Clin Pathol 1993; 46: 890-5. [ Links ]
11. Red men should go: vancomycin and histamine release. Lancet 1990; 335: 1006-7. [ Links ]











 texto em
texto em