Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Pharmacy Practice (Granada)
versión On-line ISSN 1886-3655versión impresa ISSN 1885-642X
Pharmacy Pract (Granada) vol.5 no.2 Redondela abr./jun. 2007
| Review |
Varenicline: A review of the literature and place in therapy
Heather P. WHITLEY, Krystal L. MOORMAN.
| ABSTRACT Evidence regarding the health consequences of smoking is undeniable, yet 21% of the American population continues to smoke. In addition to behavioral modifications, first-line treatment options include nicotine replacement therapies and bupropion SR. Varenicline, which was recently approved by the Food and Drug Administration (FDA), offers a novel mechanism of action for smoking cessation. This article reviews current first-line smoking cessation aids and evaluates the clinical trials pertaining to the efficacy and safety of varenicline. Additionally, the authors attempt to establish the role of varenicline in smoking cessation therapy and determine whether varenicline should be used prior to other first-line smoking cessation aids, particularly considering the lower costs of generic alternatives. At present, clinical studies have not established the efficacy of varenicline after repeated courses, following bupropion failures, or in various unstudied populations. Relatively poor study outcomes emphasize the need to provide patients with behavioral counseling throughout each quit attempt and for 1 year past the quit date. Key words: Smoking Cessation. Varenicline. Tobacco. Behavior Therapy. | RESUMEN La evidencia sobre las consecuencias sanitarias del tabaco es incontestable, aunque el 21% de la población americana continua fumando. Además de las modificaciones del comportamiento, las opciones de primera línea en el tratamiento incluyen terapias de sustitución nicotínica y bupropion SR. La varenicilina, que ha sido recientemente aprobada por la Administración de Alimentos y Medicamentos (FDA), ofrece un mecanismo de acción novedoso para el abandono del tabaco. Este artículo revisa las actuales ayudas de primera línea para la cesación tabáquica y evalúa los ensayos clínicos relativos a la eficacia y seguridad de la varenicilina. Además, los autores intentan establecer el papel del a varenicilina en el tratamiento de cesación tabáquica y determinar si la varenicilina debería ser usada antes de otras ayudas de primera línea, particularmente considerando el bajo coste de las alternativas genéricas. Ene l presente, los estudios clínicos no han establecido la eficacia del a varenicilina después de ciclos repetidos, tras fallos del bupropion, o en diversas poblaciones no estudiadas. Los resultados relativamente pobres de estudios enfatizan la necesidad de proporcionar a los pacientes consejo comportamental en cada tentativa de abandono y durante un año después de la tentativa. Palabras clave: Cesación tabáquica. Vareniclina Tabaco. Tratamiento comportamental. |
Heather P. WHITLEY, PharmD, BCPS. Department of Pharmacy Practice, Auburn University Harrison School of Pharmacy; and Department of Community and Rural Medicine, University of Alabama School of Medicine, Tuscaloosa,AL (USA).
Krystal L. MOORMAN, PharmD. Department of Pharmacy, Kaiser Permanente- Colorado Region (USA).
INTRODUCTION
Although the importance of smoking cessation is not a new concept, the use of a recently Food and Drug Administration (FDA)-approved medication is causing much discussion in the outpatient setting. Varenicline was approved by the FDA for sales in the United States on May 11, 2006, and was launched in August 2006. Since that time, the authors have experienced frequent requests for varenicline prescriptions by patients in primary care clinics. Although the clinical efficacy and usefulness of varenicline is relevant,1 a more important question, given the over-the-counter (OTC) status of many nicotine replacement therapies (NRT) and the generic manufacturing of bupropion SR, is what is the appropriate place in therapy for varenicline. After reviewing the negative consequences and addictive potential of nicotine, this article reviews the efficacy of current first-line smoking cessation aids, including varenicline and attempts to determine the appropriate place in therapy for varenicline in relation to the other products.
Impact of Smoking
Evidence regarding the devastating health consequences of smoking is undeniable. Not only is overall mortality increased up to 2-fold, but patients who smoke are at 2 to 4 times greater risk of developing cancer, cardiovascular disease, cerebrovascular disease, peripheral vascular disease, chronic obstructive pulmonary disease, and osteoporosis. Furthermore, smoking contributes to approximately 1 in 5 American deaths.2 Each year over 400,000 people die prematurely due to tobacco-related illnesses.3 The Centers for Disease Control and Prevention (CDC) identifies smoking as the single most important reversible risk factor for preventing disease and premature death. Despite the known negative impact of tobacco use, the CDC estimated that in 2005, 45.1 million (20.9%) American adults smoked which has increased from previous years.4
The economic burden of smoking-related illnesses has an equally impressive impact. Annual direct medical expenditures in 1998 reached $75.5 billion, while the annual indirect costs were approximately $81.9 billion.5 Collectively, society pays around $7.18 per pack of cigarettes smoked.5
The negative impact upon morbidity, mortality, and the economy heighten the importance of aiding every patient who desires a smoke-free life in achieving his/her goal. An estimated 70-80% of smokers report a desire to quit using tobacco,6-9 although the majority report "not thinking about quitting within the next 6 months".7 Furthermore, those who do attempt abstaining from tobacco use often relapse within the first month of being smoke-free.9,10 Less than 20% of all quit attempts remain successful at one year.6,11,12 Previous studies indicate that several quit attempts (4 to 11) are generally undertaken before becoming permanently tobacco-free.13 Annually, only 2.5% of current smokers permanently stop smoking.6,14 The relapse incidence and smoke-free duration until relapse speaks volumes to the addictive properties of nicotine.
Addictive Properties of Nicotine
Addiction is a complex behavioral phenomenon which causes a range of effects, from social to biochemical interactions.9,15,16 Tobacco contains many chemical compounds; however, nicotine is the principle addictive component.15,16 Nicotine activates the mesolimbic dopamine system within the brain, thus stimulating the reward pathway. Specifically, these affects have been linked to the ability of nicotine to bind to alpha4beta2 acetylcholine receptors in the ventral tegmental region of the brain.10 Like other addictive substances, elimination of the amplified reward pathway leads to negative feelings, behaviors, and quickly results in relapses. It is thus no surprise that the first smoking cessation pharmacologic aid was the administration of lower levels of nicotine in place of regular use.
Withdrawal Effects
Withdrawal effects are common upon nicotine discontinuation and typically present within 4 to 6 hours of nicotine deprivation in a regular smoker.10 Common withdrawal effects may include depression, irritability, anxiety, difficulty concentrating, hunger, impatience, fatigue, nervousness, restlessness, and insomnia. These symptoms usually peak within the first few days and resolve within 1 month of abstinence initiation.10 Cravings for nicotine are also frequently present, although not considered a withdrawal symptom by DSM-IV. Unconsciously, tobacco users maintain a minimum serum nicotine level in order to prevent these withdrawal symptoms while maintaining pleasure and arousal.8
Review of Pharmacologic and Nonpharmacologic Options
Behavioral Modification Therapy
Behavioral modification therapy is founded on the concept that one learns to smoke, and therefore must learn to quit. Goals of behavioral therapy are to change the behaviors and social cues that lead to smoking. Behavioral therapy should reinforce the importance of not smoking, provide practical counseling to aid in evaluating past quit attempts in efforts to improve the success of future attempts, and problem solving skills to help prevent relapses. During counseling sessions patients should be educated about the benefits of social support, as it provides positive influences and accountability.17 Most behavioral modifications are initiated prior to cessation and are extremely helpful for determining the most effective treatment plan.17 The duration of such behavioral counseling has a strong correlation with efficacy of success.17 Additionally, intensive behavioral therapy, with or without pharmacologic therapy, improves smoking cessation outcomes.10,18-20 Unfortunately, clinical experience shows that behavioral therapy is often neglected.
Pharmacotherapy
Until the late 1990s nicotine replacement therapy (NRT) was the only pharmacologic modality available to aid patients in becoming smoke-free. NRTs are available in 5 different delivery formulations; all are over-the-counter except for the inhaler and nasal spray. Although the efficacy of NRT is better than placebo, continuous abstinence rates after 12 weeks of NRT have been reported at only 20 to 24%.21 Addition of NRT to behavioral modifications improves success rates by approximately 2-fold.22 Using NRT attenuates withdrawal cravings by maintaining lower levels of plasma nicotine.23,24 The absence of withdrawal symptoms helps patients focus on nonpharmacologic therapies to achieve a smoke-free status. One particular draw back of NRT is the potential for cross abuse and addiction. Following nicotine nasal spray use, up to 43% of patients still administer the NRT after 1 year, although no longer smoking.25
Several studies have compared the efficacy of adding short-acting NRT to long-acting NRT.26-32 The long-acting NRT patch maintains a constant low level of serum nicotine, while the administration of short acting products (ie. nicotine gum, nasal spray, and inhaler) aid with breakthrough withdrawal cravings. Although the results are mixed, a Cochrane database analysis indicated that the pooled results demonstrate a modest, but statistically significant benefit for combination therapy (OR 1.42; 95%CI 1.14;1.76).22 As a result, the US treatment guidelines recommend combination therapy for those patients who experienced multiple failed quit attempts using monotherapy.17
Bupropion SR was FDA-approved in May 1997 as the first nicotine-free prescription medication to aid in smoking cessation. Use of bupropion SR, in combination with behavioral modifications, proved to be 1.2-times and 2.3-times more efficacious over the patch or behavioral modifications alone, respectively.33 The mechanism of benefit includes decreased withdrawal symptoms, predominately irritability, frustration, anxiety, difficulty concentrating, and restlessness, by increasing dopamine and norepinephrine concentrations within the mesolimbic center of the brain. Although adverse effects such as dry mouth and insomnia are generally mild and rarely affected discontinuation rates in bupropion studies, the dose-related incidence of seizures does limit the use of bupropion in some patients.34
Combination therapy with bupropion SR and NRT has also proven to be beneficial, but limited data exist. A 1999 study enrolled subjects into 1 of 3 treatment arms where they were randomized to either bupropion SR and NRT patch, bupropion SR and placebo patch, or NRT patch and placebo tablets.33 Results indicated that, although dual therapy was better than NRT alone, it was only equivalent to bupropion monotherapy. Despite the improved success bupropion SR and NRT offer, there is still clearly room for enhanced efficacy with smoking cessation aids.
Varenicline
In May 2006, the FDA approved varenicline via the fast-track approval process. Varenicline is a partial agonist of the neuronal alpha4beta2 nicotinic acetylcholine receptor, within the mesolimbic system of the brain. Nicotine reaches this location of the brain within 10 to 20 seconds after cigarette smoking.9,35 Here it binds to the alpha4beta2 nicotinic acetylcholine receptor inducing a conformational change, and allowing the influx of dopamine into the synaptic cleft.16 The drug was developed by altering the structure of (-)cytisine, a natural compound known to have partial agonist activity at the alpha4beta2 nicotinic acetylcholine receptor that has previously been studied as a smoking cessation aid.36 Like nicotine, varenicline also provides agonism to the receptor, but to a much lower degree (approximately 60% stimulation of nicotine).23 Thus, a smaller amount of dopamine is released into the synaptic cleft. By providing this low level of dopamine stimulation, varenicline aids in the attenuation of withdrawal symptoms and cravings for nicotine.
Secondly, by occupying the active site, varenicline provides competitive inhibition with nicotine to activate the alfa4beta2 receptors. Thus, nicotine is unable to activate the central nervous mesolimbic dopamine system in the presence of varenicline therapy. In turn, the neuronal mechanism underlying reinforcement and reward experienced upon smoking is blunted.37
Analysis of Varenicline Literature
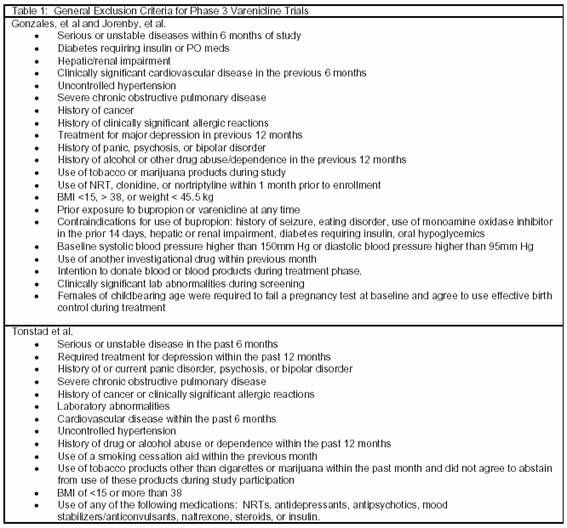
Gonzales et al (n=1025) and Jorenby et al (n=1027) conducted clinical trials evaluating the efficacy of varenicline compared with both placebo and bupropion SR. The studies had identical designs and were randomized, double-blind, placebo-controlled, multicenter trials with a 12-week treatment period and 52-week follow-up. Eligible patients were between the ages of 18 and 75 years, smoked at least 10 cigarettes per day, and had not been abstinent from cigarette smoking for longer than 3 months in the last year. Exclusion criteria are summarized in table 1. Patients were randomized to treatment with varenicline, bupropion SR, or placebo. Patients in the varenicline arm received 0.5 mg/day on days 1 to 3; 0.5 mg twice daily on days 4 to 7; and 1 mg twice daily through week 12. Patients randomized to bupropion received 150 mg/day on days 1 to 3 and 150 mg twice daily through week 12.
A target quit date was set for day 8 of the study. Patients were contacted via telephone 3 days after the quit date. In addition, patients attended weekly visits throughout the 12-week treatment phase. At each visit, brief behavioral modification counseling (10 minutes or less) was provided. Those patients who completed the 12-week treatment phase were included in the follow-up phase, which lasted from week 13 through week 52. During the follow-up phase, patients were seen in clinic approximately every 12 weeks and were evaluated via telephone on 6 additional occasions. During each contact, patients were evaluated for cigarette smoking and were provided brief counseling.
The primary endpoint in both trials was the 4-week continuous abstinence rate for weeks 9 through 12. This was defined as the proportion of subjects who reported neither smoking nor use of any nicotine-containing products, confirmed by an exhaled carbon monoxide (CO) concentration of 10 ppm or less. Secondary outcomes were continuous abstinence rates from week 9 through 24 and week 9 through 52.
Completion rates at week 52 ranged from 54 to 70%. The average age of participants was around 42 years; patients smoked an average of 22 cigarettes per day, and had been smoking for approximately 24 years. The mean Fagerström Test score, reported in the Jorenby study, was 5.4. In both trials, patients randomized to receive varenicline were 4 times (OR=3.85) and 2 times (OR=1.93 and 1.90) more likely to remain smoke-free compared with placebo and bupropion SR, respectively during weeks 9 through 12. Additionally, 80 to 85% of randomized patients had at least one previous quit attempt during which the patient may have used NRT, clonidine, or nortriptyline, thus indicating that varenicline is beneficial in the face of previous therapeutic failures with these agents.37,38
Unfortunately, there are limitations in these trials that may reduce the external validity of the data obtained. The patient population studied in all varenicline trials were predominately Caucasian men and women in their mid forties. This narrow scope of studied subjects limits the ability to extrapolate effects to other ethnicities and age groups. It is important to note that all randomized patients smoked cigarettes rather than using other tobacco-containing products. As a result, the utility of varenicline in non-cigarette tobacco users is unknown. Exclusion criteria were exceptionally broad, such that any patient with a current or prior history of a major cardiovascular-, neurologic-, or psychiatric problem was not enrolled into the studies. It is perhaps most concerning that patients at the highest risk for tobacco-related death were excluded.
Per the study design, patients were provided initial and weekly individualized counseling on behavioral modifications through week 12. Through the duration of the study (weeks 13-52) patients were still provided brief counseling on a monthly basis. Enrolled patients were required to be individuals motivated to quit using tobacco. In light of these additional nonpharmacologic therapies, it would be unreasonable to expect the majority of patients treated in the standard outpatient setting to achieve such results, as few are initially motivated or provided such extensive behavioral counseling on a regular basis. Furthermore, abstinence rates were based on patient report. Although CO measurements were used to confirm reports, this method is not a reliable means to verify smoking history for the 1 week or more that elapsed between visits, as the half-life of CO is only 4 to 5 hours.40
It should be noted that at the end of the studies, a statistically significant difference in smoking abstinence rates between patients randomized to varenicline and bupropion SR was not demonstrated consistently [p=0.57 (Gonzales), p=0.004 (Jorenby)], although the abstinence rate with varenicline was always statistically different compared with placebo [p=0.001 (Gonzales), p<0.001 (Jorenby)]. Surprisingly, Jorenby and colleagues, noted a lack of statistically significant differences between bupropion SR and placebo at the end of the study (p=0.08). This lack of difference clouds the accuracy of the bupropion arm in this study, possibly representing a type I error in the analysis between bupropion SR and varenicline.
Lastly, an average of only 20% of patients randomized to varenicline remained tobacco-free at the conclusion of the 52-week study. This emphasizes the continued high relapse rate, regardless of the pharmacologic or nonpharmacologic therapy used to aid in the initial quit attempt.
The effect of maintenance therapy with varenicline was also evaluated by Tonstad et al. This was a 52-week, multicenter trial including cigarette smokers between the ages of 18 and 75 years who smoked an average of 10 or more cigarettes per day with no abstinence period greater than 3 months. Exclusion criteria are summarized in table 1. Patients first participated in 12 weeks of open-label therapy with varenicline 0.5 mg/day on days 1 to 3; 0.5 mg twice daily on days 4 to 7; and 1 mg twice daily through week 12. Subjects who abstained from all forms of tobacco use, including nicotine replacement therapy, for at least the last 7 days of the treatment period were randomized to either varenicline 1 mg twice daily or placebo for another 12 weeks. Abstinence was confirmed by an end expiratory CO concentration of 10 ppm or less. After 12 weeks of double-blind therapy, patients were followed for an additional 28 weeks. Patients were also provided with brief smoking cessation counseling at baseline and on a weekly basis.
The primary efficacy end point was the continuous abstinence rate, confirmed by CO expiration, from week 13 through 24. Secondary outcomes included continuous abstinence from week 13 through 52 and time to first relapse.
Of the 1927 patients enrolled in the open-label phase, 1210 were eligible and agreed to participate in double-blind treatment. On average, participants were 45 years old, had a Fagerström Test score of 5.4, smoked for 27 years, and consumed an average of 22 cigarettes per day. Approximately 82% of patients made at least 1 previous attempt to stop smoking.
The continuous abstinence rate for weeks 13 through 24 was approximately 2.5 times greater with varenicline compared with placebo (OR=2.48; 95%CI 1.95;3.16 P<0.001). Results through week 52 were less impressive, but still statistically significant (OR=1.34; 95%CI 1.06;1.69 P=0.02). In order to achieve continuous abstinence in 1 patient from week 13 through 24, 5 patients would need to be treated with varenicline 1 mg, twice daily for 24 weeks. In order to achieve the same endpoint at 52 weeks, 14 patients would need to be treated. The median time to first relapse was significantly longer in the varenicline group than in the placebo groups (198 days vs. 87 days; P<0.001).41
In addition to the limitations encountered in the trials conducted by Gonzales and Jorenby, the Tonstad trial may have had issues maintaining the study blind. A common adverse effect in all varenicline clinical studies was nausea. Patients and investigators were familiar with this side effect experienced with varenicline treatment prior to entering the double-blind portion of this study. The incidence of nausea with varenicline was approximately twice that of placebo. Therefore, patients who experienced nausea in the double-blind phase may have suspected the arm to which they were randomized. Given that tolerance to this adverse effect may develop with time, this may or may not have affected the study outcome. Another limitation of the trial is the absence of an active comparator which would have made the data more applicable to clinical practice. Although this trial indicates that 12 additional weeks of varenicline therapy, at an approximate cost of $300, appears to be better than placebo it does not provide insight in quantifying efficacy of varenicline compared with bupropion. Finally, by including only patients who demonstrated some degree of success in the open-label phase of the trial, the investigators may have biased the study in favor of varenicline.
From these data, prescribers should note that varenicline is not a magic bullet. For optimal efficacy varenicline should be prescribed along with intense behavioral modification therapy that is provided at regular and frequent intervals before, during, and for a substantial period after the treatment phase.
Place in therapy for varenicline
According to the US guideline, all willing and interested patients should be offered pharmacologic therapy for smoking cessation.17 Considering this, the question that remains a challenge to practitioners is whether varenicline should be considered prior to other first-line therapies. Based on currently available literature, the appropriate place for varenicline in therapy is not clear. It is important to note that there is no apparent role for concomitant NRT and varenicline because varenicline would block the nicotine at the receptor per its mechanism of action. Published studies with varenicline contain limitations, as previously discussed, which weaken the external validity. Additionally, they do not compare varenicline to NRT and non-NRT other than bupropion. However, to aid practitioners in selecting the most appropriate agent for their patients we provide the following recommendations to help determine the appropriate place in therapy for varenicline.
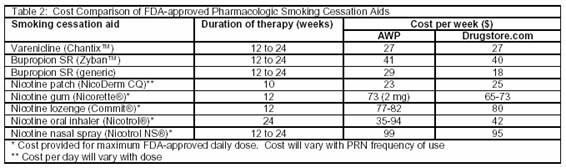
If bupropion therapy is contraindicated, the choice between varenicline and NRT is a matter of cost, compliance, and continued nicotine use. Although application of NRT provides lower plasma concentrations than cigarettes and lower health risks than continued tobacco use,42 patients who absolutely require immediate nicotine cessation may benefit greater from non-NRT. This eliminates all nicotine administration and potential for cross addiction. As most NRTs are administered multiple times per day, poor adherence may reduce success rates,23 whereas only twice daily dosing of varenicline may be more manageable from a compliance standpoint. At first glance, varenicline therapy appears more economical compared to most NRT. However, costs provided in table 2 are based on maximum FDA-approved daily doses per week. Patients frequently do not use this maximum daily allowance and may decrease the frequency of prn use over time. Therefore, the cost of NRT may be lower or equivalent to varenicline therapy. (See table 2). Additionally, NRT may be preferred in patients without contraindications due to the vast amount of experience and safety data available compared with varenicline.
The choice of pharmacologic therapy is further complicated in patients who may benefit from either oral agent, particularly considering lower cost generic equivalents. Each practitioner should consider whether the patient will benefit from the initial boost in abstinence rates seen with varenicline which is almost 2 times that of bupropion. However, this initial and unsustained benefit, which may last only 24 weeks,38 comes at an additional cost of $108 for that 3-month period compared with generic bupropion SR. The practitioner should recall that after completing a 24-week trial of either varenicline or bupropion studies showed mixed results of those remaining smoke-free at the 52-week follow-up.39 In light of this, the less expensive option of generic bupropion may be equally effective in the long-term, and thus may be a better first-line option.
The appropriate place for varenicline use in patients with several past attempts to quit, including prior use of bupropion therapy is still unclear. Patients enrolled in clinical trials had no previous exposure to bupropion; therefore, the efficacy of varenicline in patients who have previously failed bupropion SR is unknown at this time. Whether repeated courses of varenicline will yield a decrease in efficacy similar to that experienced with bupropion SR retreatment43 remains to be determined. Lastly, it is unknown whether varenicline will prove useful in treating patients who use tobacco-containing products other than cigarettes. Future studies should address these issues as well as determine the benefit of varenicline in the unstudied populations, including non-Caucasians, teenagers, the elderly, and those with cardiovascular, endocrine, or psychiatric illnesses who were excluded from varenicline clinical trials.
Regardless of when varenicline is used, it is vital to consider the important role continued education and encouragement play. Practitioners should make every attempt to ask, advise, assess, assist, and arrange as encouraged in the treatment guidelines.17 Without these important nonpharmacologic modalities, patients are not provided the best possible chance of becoming smoke-free.
CONCLUSION
Varenicline offers a novel mechanism of action in smoking cessation therapy and appears to be more effective than bupropion at week 12 in the limited population studied. Only time and experience will determine the role varenicline will play in diverse populations, after bupropion SR failures, and with repeated courses. Relatively poor study outcomes emphasize the need to provide every patient with intense behavioral counseling throughout each quit attempt and at least 1 year past the quit date.
DISCLOSURE
The authors have no financial information to disclose or any other conflict of interest.
| References |
1. Klesges RC, Johnson KC, Somes G. Varenicline for smoking cessation. Definite promise, but no panacea. JAMA 2006;296(1):94-5. [ Links ]
2. American Cancer Society. Cigarette Smoking. (revised 11-14-2003) Available on line at: http://www.cancer.org/docroot/PED/content/PED_10_2X_Cigarette_Smoking_and_Cancer.asp. Accessed November 29, 2006. [ Links ]
3. Centers for Disease Control and Prevention. Annual smoking-attributable mortality, years of potential life lost, and productivity lost – United States, 1997-2001. MMWR 2005;54(25):625-8. [ Links ]
4. Centers for Disease Control and Prevention (CDC). Tobacco use among adults – United States 2005. 2006;55(42):1146-53. [ Links ]
5. Centers for Disease Control and Prevention. Annual Smoking-Attributable Mortality, Years of Potential Life Lost, and Economic Costs --- United States, 19951999. MMWR 2002;51(14):300-3. [ Links ]
6. Benowitz NL. Nicotine addiction. Primary Care 1999;26:611-31. [ Links ]
7. Prochaska JO, DiClemente CC, Norcross JC. In search of how people change: Applications to addictive behaviors. Am Psychol 1992;47(9):1102–4. [ Links ]
8. Leave the pack behind, World no-tobacco day. World Health Organization. May 1999. [ Links ]
9. Benowitz NL.. Cigarette smoking and nicotine addiction. Med Clin North Am 1992;76(2):415-37. [ Links ]
10. Foulds J. The neurobiological basis for partial agonist treatment of nicotine dependence: varenicline. Int J Clin Pract 2006;60(5):571–6. [ Links ]
11. Balfour DJ, Fägerstrom KO. Pharmacology of nicotine and its therapeutic use in smoking cessation and neurodegenerative disorders. Pharmacol Ther 1996;72:51-81. [ Links ]
12. Schelling TC. Addictive drugs: the cigarette experience. Science 1992;255:430-3. [ Links ]
13. Smoke-free Workplaces. The World Bank. October 2003. Accessed: November 28, 2006. at: http://web.worldbank.org/WBSITE/EXTERNAL/TOPICS/EXTHEALTHNUTRITIONANDPOPULATION/EXTPHAAG/0,,contentMDK:20796948
~menuPK:1314827~pagePK:64229817~piPK:64229743~theSitePK:672263,00.html
14. US Department of Health and Human Services. Healthy people 2010: understanding and improving health. 2nd ed. Washington, DC: US Department of Health and Human Services; 2000. Available at http://www.healthypeople.gov [ Links ]
15. Dani JA, de Biasi M. Cellular mechanisms of nicotine addiction. Pharmacol Biochem Behav 2001;70:439-446. [ Links ]
16. Mansvelder HD, McGehee DS. Cellular and synaptic mechanisms of nicotine addiction. J Neurobiol 2002;53:606-17. [ Links ]
17. Fiore MC, Bailey, WC, Cohen SJ, et al. Treating Tobacco Use and Dependence. Clinical Practice Guidelines. Rockville, MD: US Department of Health and Human Services. Public Health Service. October 2000. [ Links ]
18. Foulds J, Gandhi KK, Steinberg MB et al. Factors associated with quitting smoking at a tobacco dependence treatment clinic. Am J Health Behav 2006; 30: 400-12. [ Links ]
19. Hall SM, Humfleet GL, Reus VI et al. Extended nortriptyline and psychological treatment for cigarette smoking. Am J Psychiatry 2004; 161: 2100–7. [ Links ]
20. Steinberg MB, Foulds J, Richardson DL et al. Pharmacotherapy and smoking cessation at a tobacco dependence clinic. Prev Med 2006; 42: 114–9. [ Links ]
21. Hajek P, West R, Foulds J, Nilsson F, Burrows S, Meadow A. Randomized comparative trial of nicotine polacrilex, a transdermal patch, nasal spray, and an inhaler. Arch Intern Med 1999;159:2033-8. [ Links ]
22. Silagy C, Lancaster T, Stead L, Mant D, Fowler G. Nicotine replacement therapy for smoking cessation. Cochrane Database of Syst Rev 2001;(3):CD000146. [ Links ]
23. New weapon to curb smoking. No more excuses to delay treatment. Arch Intern Med 2006;166:1547-1550. [ Links ]
24. Gourlay SG, McNeil JJ. Antismoking products. Med J Aust 1990;153:699-707. [ Links ]
25. West R, Hajek P, Foulds J, Nilsson F, May S, Meadows A. A comparison of the abuse liability and dependence potential of nicotine patch, gum, spray, and inhaler. Psychopharmacology (Berl) 2000;149:198-202. [ Links ]
26. Kornitzer M, Kittel F, Dramaix M, Bourdoux P. A double blind study of 2 mg versus 4 mg nicotine-gum in an industrial setting. J Psychosom Res 1987;31:171-6. [ Links ]
27. Puska P, Korhonen HJ, Vartiainen E, Urjanheimo EL, Gustavsson G, Westin A. Combined use of nicotine patch and gum compared with gum alone in smoking cessation: a clinical trial in North Karelia. Tobacco Control 1995;4:231-5. [ Links ]
28. Blondal T, Gudmundsson LJ, Olafsdottir I, Gustavsson G, Westin A. Nicotine nasal spray with nicotine patch for smoking cessation: randomized trial with six year follow up. BMJ 1999;318:285-9. [ Links ]
29. Bohadana A, Nilsson F, Rasmussen T, Martinet Y. Nicotine inhaler and nicotine patch as a combination therapy for smoking cessation - A randomized, double-blind, placebo-controlled trial. Arch Intern Med 2000;160:3128-34. [ Links ]
30. Tonnesen P, Mikkelsen KL. Smoking cessation with four nicotine replacement regimens in a lung clinic. Eur Respir J 2000;16(4):717-22. [ Links ]
31. Croghan GA, Sloan JA, Croghan IT, Novotny P, Hurt RD, DeKrey WL et al. Comparison of nicotine patch alone versus nicotine nasal spray alone versus a combination for treating smokers: A minimal intervention, randomized multicenter trial in a nonspecialized setting. Nicotine & Tobacco Research 2003;5(2) :181-7. [ Links ]
32. Hand S, Edwards S, Campbell IA, Cannings R. Controlled trial of three weeks nicotine replacement treatment in hospital patients also given advice and support. Thorax 2002;57(8) :715-8. [ Links ]
33. Jorenby DE, Leischow SJ, Nides MA, Rennard SI, Johnston JA, Hughes AR, et al. A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. N Engl J Med 1999;340:685-91. [ Links ]
34. Zyban (package insert). Research Triangle Park, NC: GlaxoSmithKline, DSM Pharmaceuticals, Inc., 2006. [ Links ]
35. McClure JB, Swan GE. Tailoring nicotine replacement therapy; Rationale and potential approaches. CNS Drugs 2006;20(4):281-91. [ Links ]
36. Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, et al. Varenicline: An alpha4beta2 Nicotinic Receptor Partial Agonist for Smoking Cessation. J Med Chem 2005;48:3474-77. [ Links ]
37. Chantix (package insert). New York, NY; Pfizer Labs, 2006. [ Links ]
38. Gonzales D, Rennard SI, Nides M, Onchen C, Azoulay S, Billing CB, et al. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation. JAMA 2006;296:47-55. [ Links ]
39. Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, et al. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation. JAMA 2006;296:56-63. [ Links ]
40. Stewart RD. The effect of carbon monoxide on humans. Annu Rev of Pharmacol 1975;15:409-23. [ Links ]
41. Tonstad S, Tonnesen P, Hajek P, Williams KE, Billing CB, Reeves KR. Effect of maintenance therapy with varenicline on smoking cessation. JAMA 2006;296:64-71. [ Links ]
42. McNeill A, Foulds J, Bates C. Regulation of nicotine replacement therapies (NRT): a critique of current practice. Addiction 2004;(4):CD000031. [ Links ]
43. Gonzales DH, Nides MA, Ferry LH, Kustra RP, Jamerson BD, Segall N, Buaron K, Metz A. Bupropion SR as an aid to smoking cessation in smokers treated previously with bupropion: A randomized placebo-controlled study. Clin Pharmacol Ther 2001;69:438-44. [ Links ]