Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Pharmacy Practice (Granada)
versão On-line ISSN 1886-3655versão impressa ISSN 1885-642X
Pharmacy Pract (Granada) vol.6 no.1 Redondela Jan./Mar. 2008
| Original Research |
Adverse drug reactions induced by cardiovascular drugs in outpatients
Kheirollah GHOLAMI, Shadi ZIAIE, Gloria SHALVIRI.
| ABSTRACT Considering increased use of cardiovascular drugs and limitations in pre-marketing trials for drug safety evaluation, post marketing evaluation of adverse drug reactions (ADRs) induced by this class of medicinal products seems necessary. Key words: Product Surveillance, Postmarketing. Cardiovascular Agents. Iran. | RESUMEN Teniendo en cuenta el aumento del uso de medicamentos cardiovasculares y las limitaciones en los estudios pre-comercialización, parece necesaria la evaluación de reacciones adversas (RAM) producidas por este grupo de medicamentos. Palabras clave: Vigilancia de productos post-comercialización. Agentes cardiovasculares. Irán. |
Kheirollah GHOLAMI. Professor & Chairman, Department of Clinical Pharmacy. Faculty of Pharmacy, Tehran University of Medical Sciences. Tehran (Iran).
Shadi ZIAIE. PhD. Department of Clinical Pharmacy. Faculty of Pharmacy, Tehran University of Medical Sciences. Tehran (Iran).
Gloria SHALVIRI. PharmD, MPH, Iranian Adverse Drug Reaction Monitoring Center, Undersecretary for Food and Drug Affairs, Ministry of Health, Tehran (Iran).
INTRODUCTION
The incidence of cardiovascular diseases (CVDs) has been increased in recent decades, it has been estimated that CVDs are the most common cause of death in Iran.1,2 As a result cardiovascular drugs has moved to the third place among all drug classes prescribed in the country. With introducing new cardiovascular drugs to the market, Pharmacotherapy of CVDs has improved rapidly during last few years. The problem of adverse drug events accompanied with different drug therapies has been reported since 1961. It has been reported that adverse drug events are considered as 4th to 6th cause of death in the US.3 Studies show that cardiovascular drugs are among the most commonly cause of adverse events in hospitalized patients.4 Some studies report that cardiovascular drugs may cause half of all hospital admissions due to adverse drug reactions.5 Another study describes that 4% of adverse events induced by cardiovascular drugs are serious ADEs.6 Almost 10% of all medication-related office visits result from cardiovascular drug reactions, and most of those visits are related to dermatological reactions.7 In a literature review of ten studies published between 1994 and 2001, cardiovascular drugs were implicated for 17.9% of preventable adverse drug events.8 There are several studies on hospitalized patients to detect the rate of adverse events induced by cardiovascular drugs but there are no studies on outpatients to the best of our knowledge. This is the first study evaluating adverse events following cardiovascular drugs use in outpatients.
METHODS
This cross-sectional study was conducted in the cardiovascular clinic of a 1000 bed tertiary teaching hospital in Tehran. All patients visited in the cardiovascular clinic during an eighth months period were evaluated for cardiovascular drugs induced adverse reactions. Patients previously used or newly started on cardiovascular drugs were monitored and followed for detecting and recording of ADRs. Adverse drug reactions were detected by daily interviewing patients, consulting with physicians and reviewing patient charts. The WHO definition for adverse drug reaction was used in this study: Any noxious or unintended response to a drug, which occurs at doses normally used in human for prophylaxis, diagnosis or treatment of disease or for the modification of physiological function.9
If a sign or symptom suspected to be induced by cardiovascular drug was found, the national form for ADRs (yellow card) was filled. Patient demographics, pre-existing diseases and drug history were recorded. The time of onset and duration of the reaction, suspected drug, outcome and actions taken for managing the adverse reaction were precisely recorded. The ADRs were recorded based on WHO terminology.10 Causality assessments were performed using WHO criteria.11 Seriousness of recorded ADRs were assessed based on WHO definition, which involves any ADRs resulted in death, life threatening situation, hospitalization, prolonged hospital stay, disability and birth defect.12 Preventable adverse events were determined applying Schumock questionnaire.13
All patients entered the study were classified to two different groups: Patients who developed at least one ADR (ADR patients) and patients who never experienced an ADR (Non-ADR patients). These two groups of patients were compared in sex and age by using chi-square test. Also the duration of drug usage were compared in ADR and Non-ADR patients using t-test. For assessing the relationship between frequencies of ADRs occurred and the number of drugs used, Pearson analysis was performed.
RESULTS
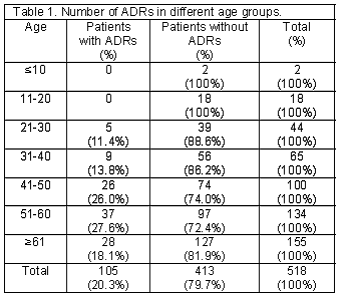
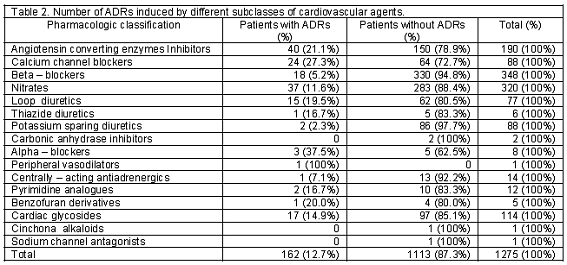
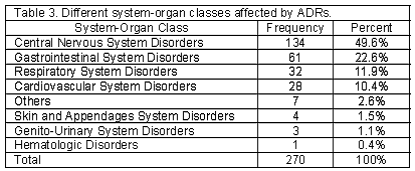
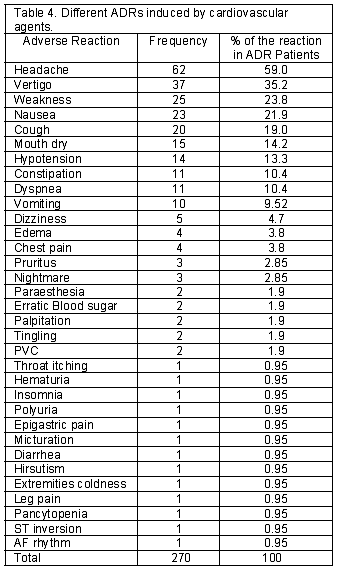
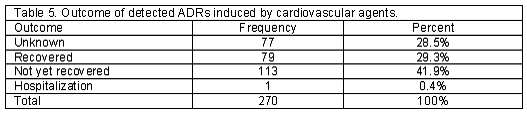
A total of 518 patients, 212 men and 306 women, using cardiovascular medications entered the study. One hundred and five patients (20.3%) including 34 men and 71 women experienced at least one ADR. There were 54 patients (51.4%) who developed more than one ADR. Two ADRs in 26 patients (24.8%), three ADRs in 21 (20%), four ADRs in 5 (4.8%), five ADRs in 1 (1%) and six ADRs in 1 (1%) patient was reported. Detected ADRs were mostly observed in the age group of 51-60 (Table 1). Calcium channel blockers and potassium sparing diuretics had the highest and lowest rate of ADRs respectively (Table 2). The highest rate of ADRs was recorded to be induced by Diltiazem (23.5%) and the lowest rate was related to Atenolol (3%). Central nervous system and Gastrointestinal system disorders were the most frequent system-organ classes affected with ADRs. (Table 3) Headache, vertigo, weakness, nausea and vomiting were the most frequent reactions. (Table 4) Among ADRs evaluated, 1.1% was recognized as serious and 1.9% as preventable ADRs. Causality assessment of ADRs revealed that the most frequent ADRs (75.9%) were recognized to be certain, followed by 19.2% as possible, 3% as probable and 1.9% as unlikely. Withdrawal of suspected drug was necessary in 22.2% of ADR patients, the treatment was continued in 65.6%, the dosage was decreased in 6.3%, the treatment was continued by alternate drug in 3.7% and 2.2% of the patients went through symptomatic therapy. The most common outcome of ADRs was not yet recovered, which refers to the ADRs not completely recovered by the end of the study (Table 5).
The result of chi-squared test for comparing sex between ADR and Non-ADR patients showed that women significantly developed more ADRs in this study (chi square = 3.978, P<0.05 ). Also the result of the chi-squared test for comparing the age groups between two groups of patients was significant, it appears that ADRs more frequently occurred with increasing age in this study (chi square = 15.871, P<0.05). Conducting t-test for comparing duration of drug usage between ADR and Non-ADR patients implicated that there is a significant relationship between two groups. It appears that the average duration of drug usage is longer in Non-ADR group (t=-2.812, P<0.05). The result of Pearson test implicated that there is a significant relationship between frequencies of ADRs occurred and the number of drugs used. It appears that with increasing the number of drugs used, the frequency of ADRs will increase (Pearson=0.259, P<0.05).
DISCUSSION
In this study 105 patients (20.3%) developed at least one ADR. This rate is higher than the rate of 15.3% previously reported in a similar study conducted in Denmark.14 This difference may be due to the difference between the population studied, types and number of drugs used by patients, definition used for ADR and the susceptibility of patients for developing adverse reactions induced by cardiovascular drugs. Most ADR patients in this study were in the age group of 51-60. This is partly in accordance with the result of a previous study conducted in Iranian hospitalized patients in two internal medicine wards in the same hospital.15 There is a controversy in the literature on the relationship between age and developing ADRs.16 Some studies have shown that ADRs may increase with increasing age; this could be due to polypharmacy used in old patients. Considering the result of chi-square test for analytical evaluation of the influence of age on occurring ADRs, it appears that older patients are more likely to experience an ADR. Gender difference in patients with or without ADRs has been described earlier17, our findings support the theory of increased number of ADRs in women.
Calcium channel blockers especially Diltiazem had the highest rate of ADRs in our study. The results of a study conducted in a university hospital showed that Nitrates, Digoxin, Propranolol, Heparin, Warfarin, Anti-hypertensive and Anti-arrhythmic drugs together produced 48.5% of ADEs. In the study performed by Mjorndal et al.18 in a clinic of internal medicine at a Swedish university hospital, cardiovascular drugs were the most common class of drugs involved in the induction of ADRs constituting 36.3% of the drugs associated with ADRs. The most offending cardiovascular drugs in that study were Metoprolol, Enalapril, Digoxin, Flodipine and Furosemide. Also in Danish trial14, Diuretics, Beta-blockers and Calcium antagonists were responsible for 80% of all ADRs detected. Our study identified Central Nervous and Gastrointestinal System as the most frequent affected system-organ classes by ADRs, in which headache, vertigo, weakness, nausea and vomiting were the most reactions observed. This profile of adverse reactions is well adjusted with the pharmacological actions of most frequent suspected drugs for ADRs in this study, where as in other study conducted in Denmark hypokalemia was the most frequent ADR related to Thiazides.
Comparing our results with those in the literature, the percentage of preventable ADRs in this study (1.9%) is rather lower than those detected in other studies.19 This may be partly due to different drug classes studied in different trials, e.g. in the similar study designed in two internal medicine wards 58.8% of detected ADRs were reported to be preventable based on the same questionnaire.15 Also in a literature review conducted on ten studies published between 1994 and 2001, cardiovascular drugs were implicated for 17.9% of preventable adverse drug events.8
This study showed that the average duration of drug usage is longer in Non-ADR group. Also it appears that most detected ADRs have been occurred shortly after starting cardiovascular drugs and incidence of ADRs are not related to the duration of usage. Like many other studies, increasing the number of drugs led to increased frequency of ADRs.
CONCLUSION
Monitoring adverse drug reactions in patients using cardiovascular drugs is a matter of importance since this class of medicine is usually used by elderly patients with critical conditions and underlying diseases. The frequency of ADRs occurrence can be reduced by decreasing the number of drugs prescribed. ADRs of Cardiovascular drugs mostly occur in first days of treatment, therefore monitoring patients in first days of using cardiovascular drugs could help in preventing ADRs. To determine the rate and nature of adverse events induced by different subclasses of cardiovascular drugs, more studies are recommended in various populations.
CONFLICT OF INTEREST
None declared. No external funding sources declared.
| References |
1. Hadi N, Rostami Gooran N. Determinant factors of medication compliance in hypertensive patients of Shiraz, Iran. Arch Iranian Med 2004;7(4):292-6. [ Links ]
2. Yazdanbod A, Nasseri-Moghaddam S, Malekzadeh R. Upper gastrointestinal cancer in Ardabil, North-West of Iran: A review. Arch Iranian Med 2004;7(3):173-7. [ Links ]
3. Lazarou J, Pomeranz BH, Corey PN. Incidence of Adverse Drug Reactions in Hospitalized Patients. A Meta-analysis of Prospective Studies. JAMA 1998;279:1200-5. [ Links ]
4. Bates DW, Cullen DJ, Laird N, Petersen LA, Small SD, Servi D, Laffel G, Sweitzer BJ, Shea BF, Hallisey R, et al. Incidence of adverse drug events and potential adverse drug events. Implications for prevention. ADE Prevention Study Group. JAMA 1995;274:29-34. [ Links ]
5. Levy M, Kewitz H, Altwein W, Hillebrand J, Eliakim M. Hospital admissions due to adverse drug reactions: a comparative study from Jerusalam and Berlin. Eur J Clin Pharmacol. 1980;17:25-31. [ Links ]
6. Zaidenstein R, Eyal S, Efrati S, Akivison L, Koren Michowitz M, Nagornov V, Golic A. Adverse drug events in hospitalized patients treated with cardiovascular drugs and anticoagulants. Pharmacoepiemiol Drug Saf 2002;11:235-8. [ Links ]
7. Frishman WH, Brosnan BD, Grossman M, Dasgupta D, Sun DK. Adverse dermatologic effects of cardiovascular drug therapy: part III. Cardiology Rev. 2002;10(6):337-48. [ Links ]
8. Kanjanarat P, Winterstein AG, Johns TE, Hatton RC, Rothi RG, Segal R. Nature of preventable adverse drug events in hospitals: A literature review. Am J Health-Syst Pharm. 2003;60:1750-9. [ Links ]
9. Edwards IR. Pharmacological basis of adverse drug reactions. In: Averys Drug Treatment (4th edn). Adis International Limited: New Zealand, 1997: 261-299. [ Links ]
10. WHO collaborating center for international drug monitoring, the Uppsala Monitoring Center. Adverse reaction terminology, 1996. [ Links ]
11. Meyboom RHB, Hekster YA, Egberts ACG, Gribnau FWJ, Edwards IR. Causal or casual? The role of causality assessment in Pharmacovigilance. Drug Saf. 1997;16:374-389. [ Links ]
12. World Health Organization. Uppsala Monitoring Center. Safety monitoring of medicinal products, guidelines for setting up and running pharmacovigilance center, Geneva, 1996. [ Links ]
13. Schumock GT, Thornton JP. Focusing on the preventability of Adverse Drug Reactions. Hosp Pharm. 1992;27:538. [ Links ]
14. Davidson F, Haghfelt T, Gram LF, Brosen K. Adverse drug reactions and drug non-compliance as primary cause of admission to a cardiology department. Eur J Clin Pharmacol. 1988;34:83-6. [ Links ]
15. Gholami K, Shalviri G. Factors associated with preventability, predictability and severity of ADRs. Ann Pharmacother 1999;33:236-240. [ Links ]
16. Lee A. Adverse Drug Reactions (2nd edition). Published by the Pharmaceutical Press. 2006; 8-10. [ Links ]
17. Rademaker M. Do women have more adverse drug reactions? Am J Clin Dermatol 2001;2:349- 351. [ Links ]
18. Mjorndal T, Boman MD, Hagg S, Backstom M, Wiholm BE, Wahlin A, Dahlqvist R. Adverse drug reactions as a cause of admissions to a department of internal medicine. Pharmacoepidemiol Drug Saf. 2002;11:65-72. [ Links ]
19. Pearson TF, Pittman DG, Longly JM, Grapes ZT, Vigliotti DJ. Mullis SR. Factors associated with preventable adverse drug reactions. Am J Hosp Pharm. 1994;51:2268-72. [ Links ]