Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Pharmacy Practice (Granada)
versão On-line ISSN 1886-3655versão impressa ISSN 1885-642X
Pharmacy Pract (Granada) vol.7 no.1 Redondela Jan./Mar. 2009
| Original Research |
General medications utilization and cost patterns in hospitalized children
Utilización general de medicamentos y patrones de costes en niños hospitalizados
Doaa OKASHA, Imad KASSIS, Salim HADDAD, Norberto KRIVOY.
| ABSTRACT Drug utilization in the in-patient setting can provide mechanisms to assess drug prescribing trends, efficiency and cost-effectiveness of hospital formularies and examine sub-populations such as children for which prescribing habits are different from adults. Key words: Drug Utilization. Inpatients. Child. Israel. | RESUMEN La utilización de medicamentos en el entorno hospitalario puede proporcionar mecanismos para evaluar las tendencias de prescripción de medicamentos, la eficiencia y coste-efectividad de los formularios hospitalarios y examinar sub-poblaciones tales como los niños para los que los hábitos de prescripción son diferentes de los adultos. Palabras clave: Utilización de medicamentos. Pacientes hospitalizados. Niño. Israel. |
Doaa OKASHA BA, Clinical Pharmacology Institute, Rambam Health Care Campus.Haifa (Israel).
Imad KASSIS MD, Senior Physician in Pediatric and Infectious Diseases, Pediatric Infectious Diseases Unit, Meyer Pediatric Hospital, Rambam Health Care Campus. B. Rappaport Faculty of Medicine, Technion -Israel Institute of Technology, Haifa (Israel).
Salim HADDAD PhD, Chief Pharmacist and Director of the Central Pharmacy, Pharmacy Services, Rambam Health Care Campus.Haifa (Israel).
Norberto KRIVOY MD, FCP, Senior Physician in Medicine and Director of the Clinical Pharmacology Institute, Rambam Health Care Campus. B. Rappaport Faculty of Medicine, Technion -Israel Institute of Technology, Haifa (Israel).
Received (first version): 22-Oct-2008
Accepted: 16-Feb-2009
INTRODUCTION
Drug utilization is an important component of many research initiatives that examine the clinical and economic effectiveness of pharmacotherapy. Monitoring medications use and knowledge of prescription habits are some of the strategies recommended for containing and controlling medication cost and its effect on national budget. Interventional programs should focus on promoting rational medication prescriptions such as the use of human albumin, antimicrobials, antivirals or hyperimmune gamma globulin (IVIG) aimed at minimizing futile expenses.
The application of drug utilization monitoring likewise provides further input into utilization correlation with medication effectiveness, prescribing habits, and time dependencies.1
A globally accepted dose standard unit is important for drug utilization (DU) studies, particularly if the investigations are performed in countries situated in different geographic areas and are to be compared.2 The DDD is a technical unit for comparison - the average recommended daily dose of a drug when used for its main indication.3 The DDD methodology was developed in response to the necessity to convert and harmonize readily available volume data (bulk costs and prescriptions) from supply statistics of pharmacy inventory data into medically meaningful units, and to make crude estimates of the number of persons exposed to a particular drug or class of drugs.2,4
Drug utilization has been defined as the prescribing, dispensing and ingesting of drugs.5 The Drug Utilization 90% (DU90%) index was introduced as a simple, inexpensive and flexible method for assessing the quality of drug prescriptions. It identifies the drugs accounting for 90% of the volume of prescribed drugs after ranking the drugs used by volume of DDD. The remaining 10% may contain specific drugs used for rare conditions in patients with a history of drug intolerance or adverse effects, complex co-morbid conditions and/or therapy prescribed by others.6
It has been recommended the DU 90% method for assessing general quality in drug prescribing2-4,7 habits, this index is a reliable cut-off level for pharmacoepidemiology and economic surveys, and can be considered for the elaboration of a health cost index.4 It has been advocated that the Drug Cost 90% Index (DC90%) should be included in drug utilization research studies.4,6,7
Drug utilization studies in the in-patient setting can provide a mechanism to assess drug prescribing trends, efficiency, and cost-effectiveness of hospital formularies and examine subpopulations for which prescribing habits may be different. In the realm of pediatric pharmacotherapy, the investigation of drug utilization is used to examine different outcomes, including the examination of prescribing trends in clinical settings, the extent to which best practices in children differ from drug monograms/labeling and adult dosing guidelines, the cost-effectiveness of hospital formularies, and the correlation between medication errors and utilization.1
The aim of this descriptive study was to analyze general medication utilization patterns and costs excluding antimicrobials prescriptions and to compare two pediatric admission units in a tertiary care university hospital.
METHODS
Rambam Health Care Campus is a 1000-bed urban tertiary care teaching hospital affiliated with the B. Rappaport Faculty of Medicine of the Technion, Israel Institute of Technology, in Haifa, Israel. In this set-up are located 2 pediatric admission units who belong to the Meyer Hospital.
Prospective data collection regarding medications used, diseases severity and patient outcome was performed in the Pediatric A and B admission units, respectively. The emergency admissions between 15:00 up to 07:59 of the next day were admitted at-random from the Pediatric Emergency Department to the admission units. Elective admission from the pediatric out-patients clinics were admitted accordingly to the following admission policy: children suffering from rheumatic or gastrointestinal diseases were admitted to the B unit. Children suffering diabetes mellitus or other endocrine diseases, lung diseases or immune deficiencies were admitted to the A unit. Other elective admissions were admitted at random to the A or B unit, respectively. The study protocol was approved by the local Ethical Commission and was registered in the NIH Clinical Trials registry with the number 00550706. Data on individual prescriptions, utilization patterns and medication costs were collected prospectively from October 15, 2007, up to April 7, 2008, in children for whom at least one medication exposure was registered.
The same observer (DO) was responsible for recording and feeding the data into the computer programs. Upon child admission, the following data were recorded on individual forms: admission date, age, gender, main admission diagnosis, medication prescriptions, delivery route, dose, starting day, and therapy ending day.
General medications were all those medications prescribed by a physician or OTC medications delivered by a nurse to admitted children. The general medications prescriptions exclude antiviral, antifungal and antimicrobial medications. The off-level indications were not taken into consideration in this study.
Exposure to a medication was considered when the child received the prescribed medication, and the nurse registered it in the individual electronic file. Prescription is the written request for a medication supply from the pharmacy.
Data collection was performed according to the prescription-point prevalence technique as described in previous publications.4,7,8 For each admission unit, the encounter with the patient file for review was once a week (Mondays) on the same day-time if the encounter day was a fest or a holyday the encounter was performed on the next working day.
The ATC-DDD classification for each drug was obtained from the WHO Guidelines.9 Medication costs were obtained from the hospital pharmacy and the computer center. Costs are presented in New Israel Shekels (NIS) (NIS1=US$ 0.27).
The data obtained from both admission units were fed into an Excel program prepared especially for the survey and to the Prizm 3.0 Graph-Pad program for further analysis.
RESULTS
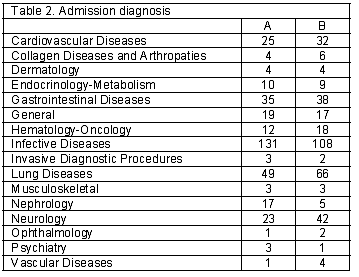
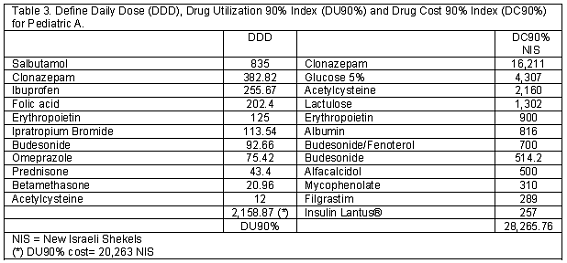
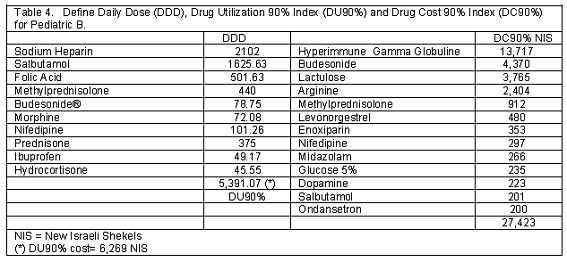
A total of 252 and 253 children were screened in this survey in Pediatric A and B. The general information data is shown in Table 1. During the study period no death were registered in both admission unit. In Table 2 the admission diagnosis is depicted. Table 3 and 4 show the DU90% and DC90% for both admission units.
In Pediatric A, salbutamol and clonazepam were the two most prescribed medications; while, in Pediatric B, sodium heparin and salbutamol were the most prescribed medications (Table 3 and 4).
No differences were depicted between the A and B units comparing the DU90% index. While, a net increment in medication cost (p=0.04) was registered according to the calculated cost from the depicted DU90% when the A (20,263 NIS) and B (6,269 NIS) admission units were compared.
Clonazepam (16,211 NIS) and glucose 5% (4,307 NIS) for the A unit and hyperimmune gamma globulin –IVIG (13, 717 NIS) and budesonide (4,370 NIS) for the B unit were the most expensive prescribed items during the study period.
DISCUSSION
In this descriptive - prospective point prevalence survey on general medication utilization pattern and cost analysis in two pediatric admission units located in a tertiary university medical center, it has been demonstrated that 94 and 88 of the surveyed individuals had at least one general medication exposure during the study periods for the A and B admission units, respectively. Salbutamol (835 DDD) and sodium heparin (2,102 DDD) were located at the head of the DU90% index list in the A and B units, respectively. Clonazepam (16,211 NIS) and hyperimmune gamma globulin (13,117 NIS) were located at the head of the DC90% index list for the respective admission A and B unit. It should be stressed that the hyperimmune gamma globulin was prescribed to only one child who suffered from Kawasaki disease.
A net increase in cost prescription attitudes was depicted when the A and B units were compared. (20,263.30 – 6,269.22 NIS) (p=0.04). The A admission unit had lower prescription volume measured by the DU90% index with higher medication cost in comparison to the B admission unit who had higher volume with lower costs. Both admission units were comparable according to admission diagnosis, children mean admission days in hospital, total admission days during the study period and total children admitted for care to each unit.
Conroy et al.10 studied 2,262 drug prescriptions that were prescribed to 624 children, 46% of all prescriptions were either unlicensed or off-label medications. In our study, using the prescription-point prevalence screening technique 187 different medications were prescribed in both admission units, 3 drugs were over the counter medications (aspirin 75 mg, paracetamol and ibuprofen) and 9 medications were off-labeled (allopurinol, amiloride, baclofen, milrinone, omeprazole, somatostatin, simvastatin, methotrexate and isoniazide) and none of the prescribed medications were unlicensed for the local legislation. There is not a wide market for many drugs in children, and drugs are expensive to be tested in this special population. For this reason, a number of drugs are used off-label in children, even though they might be of value in specialty practice.
In a recent publication by Zuppa et al.1 it had been registered 61,916 encounters in one Philadelphia's childrens hospital. Sodium chloride (9%), sodium heparin (7%), acetaminophen (5%) and albuterol (4%) were the four most prescribed medications.
The concomitant and extended utilization of two or more medications in a treatment, either due to the patients pathology or the need for action or the use of synergistic effect, is known as polypharmacy. It is estimated that those interactions occurs in 3-5% of patients treated by up to 4 different medications, and when 10 to 20 drugs are used the rate reaches more than the 20%.11 Martinbiacho J.9 found a total of 6,857 drug interactions that corresponded to 1.9 interaction/prescription.
A mean of 8 and 10 different medications / child were registered in the A and B admission units, respectively; the high medication exposures/child jeopardize those children and exposed them to potential drug-drug interactions during their in-hospital therapy.
In hospitalized adult patients the incidence of adverse drug reactions (ADR) has been widely investigated11-13; while, in children and neonates there is lack of information. The risks factor for ADR in children include multiple drug exposure with potential drug-drug interactions, complex multisystem illness, age younger than 12 months and parent or prescriber increase in dose.14
The limitations of this study were: 1) clinical outcomes were not registered; 2) children age and gender were not stratified in the results analysis; 3) the main discharge diagnosis was not taken into consideration; 4) the developed adverse drug reactions were not registered; 5) OTC medications and off-level indications were not registered.
Interventional program should be instituted for better drug prescription and utilization. In institutions fortunate enough to have clinical pharmacologist and/or a clinical pharmacist consultation services it has been established that cooperative efforts among the medical and administrative staffs should lead to early and mandatory consultations for patients with multiple organ diseases or multi drug exposures who need innovative technologies.8,15 The consultant should be involved personally when new technologies are suggested, for example: human free salt albumin for the treatment of severe hypoalbuminemia due to diffused mucositis after bone marrow transplantation or hyperimmune gamma globulin (IVIG) together with alternative plasmapheresis for the treatment of acute vascular rejection after kidney transplantation or the use of IVIG in multi-bacterial infection and severe sepsis with multi-organ failure.
It has been recommended 1 the creation of a local, national or even a global drug utilization network to facilitate the examination of geographical and /or socio-economic influences in drug utilization and prescribing practices in general and for children in special clinical settings.
CONCLUSIONS
A significant difference in the prescription number of general medications volume/patient (8.4-10.9; p=0.02) was depicted between the A and B admission units
Increased cost in prescription attitudes was depicted in the A unit in comparison to the B unit (20,263.30 – 6,269.22 NIS) (p=0.04). The A admission unit had lower prescription volume with higher medication cost for the study period in comparison to the B admission unit.
Interventional program should be instituted for better drug prescription, medications utilization control and cost containment including also the establishment of a consultation service staffed by a clinical pharmacologist and/or a clinical pharmacist.
CONFLICT OF INTEREST
None declared.
| References |
1. Zuppa A, Vijayakumar S, Jayaraman B, Patel D, Narayan M, Vijayakumar K, Mordick JT, Barrett JS. An informatics approach to assess pediatric pharmacotherapy: Design and implementacion of a hospital drug utilization system. J Clin Pharmacol. 2007;47:1172-1180. [ Links ]
2. Matuz M, Benko R, Doro P, Hajdu E, Nagy G, Nagy E, et al. Regional variations in community consumption of antibiotics in Hungary, 1996-2003. Brit J Clin Pharmacol. 2005;61:96-100. [ Links ]
3. Guidelines for ATC classifications and DDD assignment. WHO Collaborating Center for Drug Statistic Methodology. Oslo (www.whocc.no), date of access October 1st 2007. [ Links ]
4. Krivoy N, Mattalon N. Antimicrobial utilization pattern in a hematologic intensive care unit. J Pharm Technol. 2001;17:15-18. [ Links ]
5. Bergman U, Popa C, Tomson Y, Wettermark B, Einarson TR, Aberg H, Sjoqvist F. Drug Utilization 90% - A simple method for assessing the quality of drug prescribing. Eur J Clin Pharmacol. 1998;54:113-118. [ Links ]
6. Wettermark B, Pehrsson A, Jinnerot D, Bergman U. Drug utilization 90% profiles - a useful tool for quality assessment of prescribing in primary health care in Stockholm. Pharmacoepidemiol Drug Saf. 2003; 12: 499-510. [ Links ]
7. Krivoy N, Abed El-Ahal W, Bar- Lavie Y, Haddad S. Antibiotic prescription and cost patterns in a general intensive care unit. Pharm Pract (Internet). 2007;5:67-73. [ Links ]
8. Gendel I, Azzam ZS, Braun E, Levy Y, Krivoy N. Antibiotic utilization prevalence: prospective comparison between two medical departments in a tertiary care university hospital. Pharmacoepidemiol Drug Saf. 2004;13:735-739. [ Links ]
9. Martinbiacho J, Zuckermann J, Dos Santos L, Silva MM. Profile of drug interactions in hospitalized children. Pharm Pract (Internet). 2007;5:157-161. [ Links ]
10. Conroy S, Choonara I, Impicciatore P, Mohn A, Arnell H, Rane A, et al. Survey of unlicensed and off label drug use in paediatric wards in European countries. BMJ. 2000;320:79-82. [ Links ]
11. Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients. JAMA. 1998;279:1200-1205. [ Links ]
12. Arulmani R, Rajadran SD, Suresh B. Adverse drug reaction monitoring in a secondary care hospital in South India. Br J Clin Pharmacol 2007;65:210-216. [ Links ]
13. Ortega A, Aguinagalde A, Lacasa C, Aquerreta I, Fernandez-Benitez M, Fernandez LM. Efficacy on an adverse reaction electronic reporting system integrated into a hospital information system. Ann Pharmacother. 2008;42:1491-1496. [ Links ]
14. Knight M. Adverse drug reactions in neonates. J Clin Pharmacol. 1994;34:128-135. [ Links ]
15. Byl B, Clevenbe4rgh P, Jacobs F, Struelens MJ, Zech F, Kentos A, Thys JP. Impact of infectious disease specialist and microbiological data on the appropriateness of antimicrobial therapy for bacteremia. Clin Infect Dis. 1999;29:60-66. [ Links ]