Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Pharmacy Practice (Granada)
versión On-line ISSN 1886-3655versión impresa ISSN 1885-642X
Pharmacy Pract (Granada) vol.7 no.2 Redondela abr./jun. 2009
| Original Research |
Impact of pharmacist's interventions on cost of drug therapy in intensive care unit
Impacto de las intervenciones de farmacéuticos en el coste del tratamiento farmacológico en una unidad de cuidados intensivos
Surasak SAOKAEW, Sirada MAPHANTA, Pornchanok THANGSOMBOON.
| ABSTRACT Pharmacist participation in patient care team has been shown to reduce incidence of adverse drug events, and overall drug costs. However, impact of pharmacist participation in the multidisciplinary intensive care team on cost saving and cost avoidance has little been studied in Thailand. Key words: Cost Savings. Pharmacists. Intensive Care Units. Thailand. | RESUMEN La participación del farmacéutico en el equipo de cuidados del paciente ha demostrado reducir la incidencia de eventos adversos medicamentosos, y los costes totales de medicamentos. Sin embargo, el impacto de la participación del farmacéutico en equipos multidisciplinarios de cuidados intensivos sobre el ahorro y la evitación de costes en Tailandia ha sido poco estudiado. Palabras clave: Ahorro de costes. Farmacéuticos. Unidades de cuidados intensivos. Tailandia.
|
Surasak SAOKAEW. PharmD. School of Pharmacy. Naresuan University Phayao. Phayao (Thailand).
Sirada MAPHANTA. PharmD, BCPS. Department of Pharmacy Practice. Faculty of Pharmaceutical Sciences, Naresuan University. Phitsanulok (Thailand).
Pornchanok THANGSOMBOON, MSc. Department of Pharmacy, Bhuddachinaraj Hospital. Phitsanulok (Thailand).
Received (first version): 1-Oct-2008
Accepted: 15-Mar-2009
INTRODUCTION
The role of pharmacist in health care delivery continues to evolve beyond dispensing and directly related activities.1,2 Numerous studies has been shown pharmacist make valuable contributions to improve clinical, economic, and humanistic patients' outcomes.3-29 Furthermore, many studies have evaluated the role of the pharmacist especially in intensive care unit (ICU).4,6-9,20,22,23,26 The rationale for putting a pharmacist in an ICU is that those patients are sicker and thus require a greater complexity of care. Having a pharmacist on a team in an ICU has been shown to reduce the incidence of adverse drug events (ADEs)4,25,29 and decrease costs associated with care.3,22,23,25,27-29 However, the study of the impact of pharmacist's interventions in intensive care unit in Thailand are limited.
As a result, we conducted a study of the effectiveness of pharmacist participation in a multidisciplinary team in medical ICU to provide optimal patient care through avoidance or treatment of drug related problems. The objectives of the study were to determine the type and quantity of patient care interventions recommended by a pharmacist and to specifically examine cost saving and cost avoidance that resulted from pharmacist recommendations in medical ICU.
METHODS
Setting, design, and sample
The study carried out in 2 medical ICUs at Buddhachinaraj Hospital, a large tertiary care hospital (940-bed size hospital), in Phitsanulok province, Thailand, during February 28, 2005, through April 1, 2005. There are 20 beds in each unit. Patients were admitted to ICU based on disease severity and physician's judgment for appropriated care.
Both of two units were included in the study, one as the intervention group and the other as the control group. Patients had equal chance of being admitted to the control group or the intervention group. The admitting process was based on the availability of beds and physician service. The demographics and Acute Physiology and Chronic Health Evaluation (APACHE) scores in both groups were compared to determine if they were similar. We compared outcome, direct drug cost and length of ICU stay (LOS), between the study group and control group during the same period. For the study group, we calculated cost saving and cost avoidance resulted from pharmacist intervention. This study was approved by the Institutional Review Board/Human Subject Research Committee at Buddhachinaraj Hospital.
Pharmacist's activities and interventions
The treatment team consisted of a medical intensive care team plus a clinically trained pharmacist with a Pharm.D degree. Before morning rounds each day, the pharmacist reviewed all patient profiles and relevant data, including the physician's orders, laboratories, progress and consultation notes as appropriate, and formulated plans for modifying individual patient regimens. The pharmacist then participated in morning rounds. This provided an opportunity for the pharmacist to evaluate the treatment and suggest changes in patient drug regimens. After rounds, both newly admitted patients and patients previously admitted to the ward were discussed. The pharmacist suggestions for modifying therapy were presented. The pharmacist was full responsibility for providing drug information, pharmacotherapeutic consultation, and relevant as needed for five days a week. During 8.00 a.m. to 5.00 p.m. of the day, pharmacist maintained contact with the team physicians as needed. In all case, the pharmacist aimed to provide optimal patient care through avoidance or treatment of drug related problem, base on literature. All interventions were documented in the data collecting form.
Data analysis
To perform analysis of the result, each intervention was assigned to an intervention category. The categorization of interventions was then verified by the clinical pharmacists. Since there is no standardized method of categorizing interventions made by pharmacist, the interventions were assigned to the categories used by Leape and colleagues4 in an intervention illustrating the benefit of a pharmacist in an ICU unit. The interventions that accepted and implemented by team were also categorized as either cost saving and/or cost avoidance.
Cost saving
Intervention that resulted in drug treatment with a lower or higher direct drug cost was evaluated in term of cost saving. Cost saving were calculated as the difference in actual costs between the previous therapy and the new therapy that was recommended by the pharmacist. Labor cost, supplies cost, or other indirect costs were not included in any calculation.3,22,23 The saving resulting from the change in drug therapy was generally assumed to extend to the end of therapy with the new agent.3,11,22,30 In the case of conversion from intravenous (i.v.) to oral dosage forms, the cost difference between the dosage forms was calculated for 2 days after the switch (it assumed that without the pharmacist's interventions, physician would have switched to oral dosage forms within 2 days).11,22
The formula we used to estimate cost saving is in common use equation11,23: Cost difference in USD = (cost of drug therapy multiple by frequency per day and multiple by duration of therapy, assumed to extend to the end of therapy with the new agent, before intervention) minus (cost of drug therapy multiple by frequency per day and multiple by duration of therapy after intervention plus cost of drug that was used before intervention).
Example 1. A patient was receiving ceftazidime (1 g every 8 hrs) to treat Pseudomonas aeruginosa. Three days later, patient's renal function was worsening; calculated creatinine clearance was 16.6 ml/min. The pharmacist then gave team an intervention. After intervention, the order was changed, base on renal guideline, to ceftazidime (1 g every 24 hrs). The duration of therapy was 7 days. The cost of each 1 g-vial of ceftazidime was 1.7 USD. So, the cost for this case is, (1.7 USD/1 g-vial x 3 times/day x 7 days) - [(1.7 USD/1 g-vial x 1 time/day x 4 days) + (1.7 USD/1 g-vial x 3 times/day x 3 days)], 13.6 USD. It means 13.6 USD decreased or saved.
Example 2. A patient with underlying diabetes whose blood was sent to measure electrolyte and trace element. When the result came back, pharmacist screened and saw the potassium concentration result was 8 mEq/L (K=8 mEq/L). The pharmacist then gave team an intervention. After intervention, physician ordered Kayexalate 30 g every 4 hrs for 4 doses. The cost of 30 g-powder packed Kayexalate is 4.7 USD. Thus, the cost for this case is, (4.7 USD/30 g-powder packed Kayexalate x 4 doses), 18.8 USD. It means 18.8 USD increased. In contrast, this intervention, trough monitoring potassium and prevention of serious cardiac complications and subsequent admission or may be death, was calculated as cost avoidance as well.
The net cost saving over the duration of study period was calculated by subtracting the total decrease in drug costs from the total increase in drug costs.
Cost avoidance
The intervention with potential to avoid an adverse drug events (ADE) were assessed for cost avoidance by a member panel of clinical specialists.28-30 The panel comprised seven members. Four members with doctor of pharmacy degree, three of the seven members had completed master's degree and had been experienced in ADR center for many years, and one of them was a clinical pharmacy specialist instructor who holds the Ph.D. degree and Board Certified Pharmacotherapy Specialist.
The panel evaluated each intervention to estimate the probability, in the absence of the intervention, of an ADE occurring on the basis of the clinical details surrounding the intervention. The probability of an ADE in the absence of the intervention was set at 0 (zero; e.g. information requested), 0.01 (very low; for problem orders e.g. clarifications, missing information, nonexistent strengths), 0.1 (low; for prevented a potentially significant reaction e.g. 2-4 x normal dose, dose inadequate to produce therapeutic effect; incorrect schedule/route with potential for therapeutic failure/toxicity; duplicate therapy with potential for additive toxicity), 0.4 (medium; for prevented a potentially serious reaction e.g. allergy to drug ordered, no allergy information, 4-10 x normal dose; no adjustment of renal failure), 0.6 (high; for prevented a potentially fatal or severe reaction e.g. 10 x normal dose; narrow therapeutic range; life-threatening reaction/anaphylaxis).11,30 Literature was used to assign probability estimates, when available. When no literature estimate was available, judgment based on patient's clinical data was used to assign the intervention to a probability category.30 We assumed that no intervention would increase the likelihood of a preventable ADE. The cost of each adverse event was set at 53 USD on the basis of average cost of adverse drug reaction form the previous trial.31 Cost avoidance was calculated for each intervention by multiplying the estimated probability of an ADE in the absence of the intervention with the average cost of an ADE (53 USD).29,30
Example: A patient with pneumonia was receiving amoxicillin/clavulanate potassium (1000 mg/ 200 mg every 8 hrs) to treat Stapphylococcus aureus. The pharmacist calculated creatinine clearance; it was 12.5 ml/min. The pharmacist then gave team an intervention. After intervention, the order was changed, base on renal guideline, to amoxicillin/clavulanate potassium (1000 mg/ 200 mg every 12 hrs). The panel estimated the probability of an ADE, in the absence of the intervention, was 0.4 (medium). So, the cost avoidance of this case is, (0.4 x 53), 21.2 USD.
The net change in drug costs over the duration of study period calculated by cost saving plus cost avoidance. All cost in Thai baht (THB) was converted to US dollars (1 USD about 38.36 THB).
Statistical analysis
Patient variables between the study and control group were compared using chi-square analysis for sex and Mann-Whitney U test for age, APACHE score, direct drug cost, and length of stay (LOS).32 The level significance was set at 0.05.
RESULTS
There were 65 patients admitted to the medical ICU during the study days. There was not significantly different with respect to age, sex, and APACHE score between study and control groups (Table 1).
In term of direct drug cost per capita, it was 1,076.37 USD in study group compared with 1,258.38 USD in control group. The difference of direct drug cost between two group was 182.01 USD, but did not statistical significant (p=0.138). The average LOS was 7.16 days (SD 6.62) in the study group and 6.18 days (SD 3.79) in the control group (p=0.995) (Table 2).
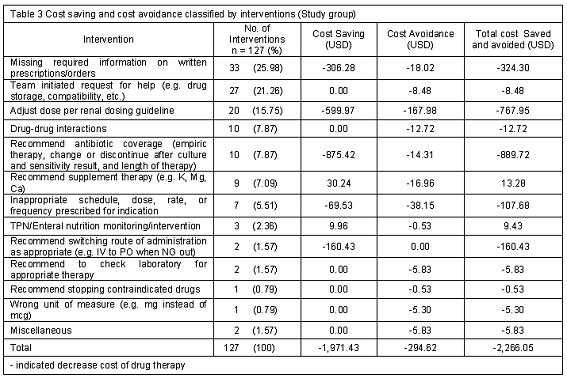
Of the 127 interventions provided by pharmacist, 125 interventions accepted and implemented by team (98.4%). These interventions were categorized and analyzed as either cost saving and/or cost avoidance. Interventions made by pharmacist resulted in direct cost saving of 1,971.43 USD and cost avoidance of 294.62 USD during study period. The total cost saving and cost avoidance was 2,266.05 USD (Table 3). The most common intervention was "providing required information on written orders" followed by "the team initiated for help and adjust dose per renal dosing guideline" (Table 3).
In addition, cost saving and cost avoidance were classified by drug class. We found that anti-infective was the major cost reduction (1,958.61 USD) followed by anticoagulants (132.36 USD). The most common drugs used in ICU were anti-infective, cardiovascular drugs, electrolytes trace elements and fluid, and anticoagulants, respectively, which required more interventions than other drugs (Table 4).
DISCUSSION
The number of adverse drug events and the subsequent cost of these events can be reduced by pharmacist intervention.3-5,10-18 In the current study, we aims to describe the characteristics of the interventions and to determine pharmacist's interventions led to change in cost saving and cost avoidance in an intensive care unit (ICU). This study documented a role for a pharmacist in a multidisciplinary ICU, and demonstrated a substantial reduction in drug costs as a result of pharmacist - initiated therapeutic consultations / interventions.
Critically ill patients with multiple system disease require multiple drug therapy; in addition, changes in their pharmacokinetic and pharmacodynamic variables due to multiple organ dysfunctions can affect the absorption, metabolism, elimination, and interaction of drugs. Thus, critical ill patients present unique pharmacologic challenges to the clinician. Although these challenges may be similar to those in general or surgical patients, their frequency and importance are increased in the ICU population.23
Monitoring for adverse drug reactions was an importance responsibility of the pharmacist. Many medications taken by ICU patients have significant adverse effect profiles and multiple known drug-drug interactions.
In addition to direct patient care activities, the pharmacist provided in-service education to the team, participated in research, and identified indigent patients and enrolled them in medication assistance programs. Other professionals on the team indicated that the direct (e.g. pharmacotherapy recommendations) and indirect (e.g. drug information) services provided made the pharmacist a valuable member of the team.
In our study documented that the 98% percent of the pharmacist's interventions were accepted and implemented by team exceeds the overall 95% acceptance rate (8.7% accepted with changing therapy) previously reported for pharmacists' recommendations.20 The quality and importance of the pharmacist's interventions are reflected in the independent evaluation of potential impact on patient care. Similarly favorable results have been reported in other centers despite some variability in the calculation of savings. For example, Bearce and colleagues33 multiplied the cost difference per day by the number of days that the patient was in ICU, regardless of whether the patient remained on the drug in question for the entire stay or not. Warrian and Irvince-Meek34 multiplied the cost difference per day by the average length of stay. However, calculating the cost difference based on the number of days the patient was receiving therapy fails to capture the benefit of a consultation that led to discontinuing a drug-one cannot know how many days this drug would have been continued if the consultation were not made. We believe that our approach to drug cost is as reasonable as others employed.
There are limitations to the conclusion of this study. First, the crude cost calculations do not include the pharmacist's time spent in chart review and making rounds with the team to identify potentially meaningful interventions. Furthermore, according to the pilot study, the sample size in this study was not calculated and got very small size that reflect the fact that there is not enough statistical power to detect the significant different between two groups. However, the results in cost saving and cost avoidance in the study group tend to be reduced. In addition, it is likely that not every pharmacist would demonstrate the same skill level as the others. Thus, these finding may not necessarily be extrapolated to other clinical pharmacist in other institutions.
Our study demonstrated that the participation of a clinical pharmacist in patient care rounds, chart review, and providing interventions resulted in benefits in term of cost saving and cost avoidance. These results suggest that a dedicated clinical pharmacist in ICU by providing interventions is likely to decrease total drug cost and play a crucial role on the ICU team.
CONCLUSIONS
Our results indicated that a pharmacist's interventions in intensive care unit had a positive potential impact on overall drug cost, cost saving, and cost avoidance and were well accepted by the team. The need to further develop the methodology to estimate the cost will be a major challenge. More studies are needed in this field.
ACKNOWLEDGEMENTS
We would like to thank the member panel; Arom Jedsadayanmata, Pharm.D, Ph.D, BCPS, Daranee Chiewchantanakit, M.Sc, Chuanchom Thananithisak, Pharm.D, Nantawarn Kitikannakorn, Pharm.D, Athagran Nakham, Pharm.D, Rungluk Kidgoukarun, M.Sc, Nophawan Jianpeerapong, M.Sc, an intensive care team; Noppadol Wanichakorn, M.D, Noppakao Kongtal, M.D, Poonsub Vithayanant, BN.S, M.Ed., Panya Theunduang, BN.S, MN.S, Prapaipan Buaon, BN.S, Nataya Kamsawang, BN.S, Duangrut Thimsri, BN.S, Siriwan Pansarp, BN.S, Kanjana Jujun, BN.S, Khunthong Chatsing, BN.S, Panita Naneprom, BN.S, Wallapha Nuchpo, BN.S, Umpai Homsaard, BN.S, Suwimol Muangpruan, BN.S, Charinun Chanyamsong, BN.S, Chutisorn Kraitip, BN.S, Aunchalee Kaewsooksai, BN.S, Sawang Panpua, BN.S, Chanakard Kaewmanee, BN.S, Manita Suaphue, BN.S, and all component authorities of the Buddhachinaraj Hospital for their assistance in the study.
CONFLICT OF INTEREST
None declared.
| References |
1. Hepler CD, Strand LM. Opportunities and responsibilities in pharmaceutical care. Am J Hosp Pharm. 1990;47:533-543. [ Links ]
2. Schiff GD, Klass D, Peterson J, Shah G, Bates DW. Linking laboratory and pharmacy: opportunities for reducing errors and improving care. Arch Intern Med. 2003;163:893-900. [ Links ]
3. Krupicka MI, Bratton SL, Sonnenthal K, Goldstein BG. Impact of a pediatric clinical pharmacist in the pediatric intensive care unit. Crit Care Med. 2002;30:919-921. [ Links ]
4. Leape LL, Cullen DJ, Clapp MD, Burdick E, Demonaco HJ, Erickson JI, Bates DW. Pharmacist participation on physician rounds and adverse drug events in the intensive care unit. JAMA 1999;282:267-270. [ Links ]
5. Schommer JC, Wenzel RG, Kucukarslan SN. Evaluation of pharmacists' service for hospital inpatients. Am J Health-Syst Pharm. 2002;59:1632-1637. [ Links ]
6. American Society of Health-System Pharmacist. ASHP guideline on the pharmacist's role in the development, implementation, and assessment of critical care pathways. Am J Health-Syst Pharm 2004;61:939-945. [ Links ]
7. Brilli RJ, Spevetz A, Branson RD, Campbell GM, Cohen H, Dasta JF, Harvey MA, Kelley MA, Kelly KM, Rudis MI, St Andre AC, Stone JR, Teres D, Weled BJ; American College of Critical Care Medicine Task Force on Models of Critical Care Delivery. The American College of Critical Care Medicine Guidelines for the Defintion of an Intensivist and the Practice of Critical Care Medicine. Critical care delivery in the intensive care unit: defining clinical roles and the best practice model. Crit Care Med. 2001:29:2007-2019. [ Links ]
8. Society of Critical Care Medicine, American College of Clinical Pharmacy. Position paper on critical care pharmacy services. Pharmacotherapy. 2000;20(11):1400-1406. [ Links ]
9. Kirk JK, Michael KA, Markowsky SJ, Restino NR, Zarowitz BJ. Critical pathways: the time is here for pharmacist involvement. Pharmacotherapy. 1996;16(4):723-733. [ Links ]
10. Kucukarslan SN, Peters M, Mlynaerek M, Nafziger DA. Pharmacist on rounding teams reduces preventable adverse drug events in hospital general medicine units. Arch Intern Med. 2003;163:2014-2018. [ Links ]
11. Suseno M, Tedeski L, Kent S, Rough S. Impact of documented pharmacists' interventions on patient care and cost. Hosp Pharm. 1998;33:676-681. [ Links ]
12. Chisholm MA, Vollenweider LJ, Mulloy LL, Jagadeesan M, Wade WE, Dipiro JT. Direct patient care services provided by a pharmacist on a multidisciplinary renal transplant team. Am J Health-Syst Pharm. 2000;57:1599-1601. [ Links ]
13. Anderson RJ. Cost analysis of a managed care decentralized outpatient pharmacy anticoagulation service. J Manag Care Pharm. 2004;10(2):159-165. [ Links ]
14. Lim WS, Low HN, Chan SP, Chen HN, Ding YY, Tan TL. Impact of pharmacist consult clinic on hospital-based geriatric outpatient clinic in Singapore. Ann Acad Med Singapore. 2004;33:220-227. [ Links ]
15. Boyko WL, Yurkowski PJ, Ivey MF, Armitstead JA, Robberts BL. Pharmacist influence on economic and morbidity outcomes in a tertiary care teaching hospital. Am J Health-Syst Pharm. 1997;54:1591-1595. [ Links ]
16. Hatoum HT, Hutchinson RA, Witte KW, Newby GP. Evaluation of the contribution of clinical pharmacists: inpatient care and cost reduction. Drug Intell Clin Pharm. 1988;22:252-259. [ Links ]
17. Zimmerman CR, Smolarek RT, Stevenson JG. A computerized system to improve documentation and reporting of pharmacists' clinical interventions, cost saving, and workload activities. Pharmacotherapy. 1995;15(2):220-227. [ Links ]
18. Gandhi PJ, Smith BS, Tataronis GR, Maas B. Impact of pharmacist on drug costs in a coronary care unit. Am J Health-Syst Pharm. 2001;58:497-503. [ Links ]
19. Schumock GT, Meek PD, Ploetz PA, Vermeulen LC. Economic evaluations of clinical pharmacy services 1988-1995. Pharmacotherapy. 1996;16(6):1188-1208. [ Links ]
20. Zaidi STR, Hassan Y, Postma MJ, Ng SH. Impact of pharmacist recommendations on the cost of drug therapy in ICU patients at a Malaysian hospital. Pharm World Sci. 2003;25(6):299-302. [ Links ]
21. McMullin ST, Hennenfent JA, Ritchie DJ, Huey WY, Lonergan TP, Schaiff RA, Tonn ME, Bailey TC. A prospective, randomized trial to assess the cost impact of pharmacist-initiated interventions. Arch Intern Med. 1999;159:2306-2309. [ Links ]
22. Chuang LC, Sutton JD, Henderson GT. Impact of a clinical pharmacist on cost saving and cost avoidance in drug therapy in an intensive care unit. Hosp Pharm. 1994;29(3):215-218. [ Links ]
23. Montazeri M, Cook DJ. Impact of a clinical pharmacist in a multidisciplinary intensive care unit. Crit Care Med. 1994;22:1044-1048. [ Links ]
24. Brockmiller H, Abel SR, Koh-Knox CP, Birk CW. Cost impact of Pharm.D. candidates' drug therapy recommendations. Am J health-Syst Pharm. 1999;56:882-884. [ Links ]
25. Kane SL, Weber RJ, Dasta JF. The impact of critical care pharmacists on enhancing patient outcomes. Intensive Care Med. 2003;29:691-698. [ Links ]
26. Ibrahim KH, Gunderson B, Rotschafer JC. Intensive care unit antimicrobial resistance and the role of the pharmacist. Crit Care Med. 2001;29(suppl):N108-N113. [ Links ]
27. Pasquale TR, Komorny KM, Letting-Mangira D, Peshek S. A pharmacist-physician antibiotic support team. P & T. 2004; 29:33-40. [ Links ]
28. Mutnick AH, Sterba KJ, Peroutka JA, Sloan NE, Beltz EA, Sorenson MK. Cost saving and avoidance form clinical interventions. Am J Health-Syst Pharm. 1997;54:392-396. [ Links ]
29. Lee AJ, Boro MS, Knapp KK, Meier JL, Korman NE. Clinical and economic outcomes of pharmacist recommendations in a Verterans Affairs medical center. Am J Health-Syst Pharm. 2002;59:2070-2077. [ Links ]
30. Nesbit TW, Shermock KM, Bobek MB, Capozzi DL, Flores PA, Leonard MC, Long JK, Militello MA, White DA, Barone LD, Goldman MP, Kvancz DA. Implementation and pharmacoeconomic analysis of a clinical staff pharmacist practice model. Am J Health-Syst Pharm. 2001;58:784-790. [ Links ]
31. Boonsrarakpong N, Thadapak U, Chariyapongphaiboon P, Kidgoukarun R, Rattanawaraha K, Preechakul S, et al. Payment of adverse drug reaction in medical ward. Thai Northern Regional Hospitals Report, 2004. [ Links ]
32. Rosner B, editor. Fundamentals of biostatistic. 4th ed. Belmont, CA: Wadsworth; 1995. [ Links ]
33. Bearce WC, Willey GA, Fox RL, et al. Documentation of clinical interactions: Quality of care issues and economic considerations in critical care pharmacy. Hosp Pharm. 1988;23:544-548. [ Links ]
34. Warrian K, Irvine-Meek J. Cost-benefit of clinical services integrated with a decentralized unit dose system. Can J Hosp Pharm. 1988;41:109-121. [ Links ]