My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Pharmacy Practice (Granada)
On-line version ISSN 1886-3655Print version ISSN 1885-642X
Pharmacy Pract (Granada) vol.10 n.1 Redondela Jan./Mar. 2012
ORIGINAL RESEARCH
Analysis of potential interactions between warfarin and prescriptions in Estonian outpatients aged 50 years or more
Análisis de las interacciones potenciales entre warfarina y medicamentos prescritos en pacientes ambulatorios mayores de 50 años en Estonia
Maia Gavronski1, Sirpa Hartikainen2, Alexander Zharkovsky3
1PhD candidate. Department of Pharmacology, Centre of Excellence for Translational Medicine, Medical Faculty, University of Tartu. Tartu, (Estonia).
2Professor of Geriatric Pharmacotherapy. School of Pharmacy, University of Eastern Finland. Kuopio (Finland).
3Professor of Pharmacology and Drug Toxicology. Department of Pharmacology, Centre of Excellence for Translational Medicine, Medical Faculty, University of Tartu. Tartu (Estonia).
This research was supported by the European Union through the European Regional Development Fund of Estonia and Estonian Research Foundation Grant No. 7955.
ABSTRACT
In Estonia, warfarin is widely prescribed by general practitioners to prevent and treat thromboembolic diseases. To date, there has been no systematic analysis of the potential risk of warfarin interactions with other drugs in the outpatient population.
Objective: The aim of the study was to analyze the incidence of potential interactions in prescription schemes in Estonia in a cohort of outpatients receiving warfarin treatment.
Methods: The retrospective study population included 203,646 outpatients aged 50 years or older of whom 7,175 received warfarin therapy. Patients who had used at least one prescription drug for a minimum period of 7 days concomitantly with warfarin were analyzed. Potential drug interactions were analyzed using Epocrates online, Stockley´s Drug Interactions and domestic drug interaction databases.
Results: The average number of drugs used concomitantly with warfarin was 4.8 (SD=1.9) (males: 4.7 SD=2.0, females: 4.9 SD=2.0). No potential interactions in treatment regimens were found in 38% of patients, one potential interaction was observed in 29% and two or more potential interactions were observed in 33% of patients. The mean number of all potential interactions was 1.2 per patient and about the same in men and women. Potential interactions were associated with the number of drugs. Warfarin-related interactions were detected in 57% of patients, and the number of interactions related to warfarin per patient varied from 1 to 5. Most frequent were use of warfarin with NSAIDs (14%), followed by simvastatin (9%) and amiodarone (7%).
Conclusion: This study shows that 57% of outpatients in Estonia receiving warfarin have drugs potentially interacting with warfarin in their treatment schemes. Most interactions (14%) with warfarin are associated with the prescription of NSAIDs.
Key words: Warfarin. Drug Interactions. Outpatients. Estonia.
RESUMEN
En Estonia, la warfarina es prescrita por médicos generales para tratar enfermedades tromboembólicas. Hasta la fecha, no se ha realizado un análisis sistemático del riesgo de interacciones potenciales de la warfarina con otros medicamentos en la población ambulatoria.
Objetivo: El objetivo de este estudio fue analizar la incidencia de interacciones potenciales en los esquemas prescritos en Estonia en una cohorte de pacientes ambulatorios recibiendo warfarina.
Métodos: El estudio retrospectivo incluyó 203.646 pacientes ambulatorios de 50 o más años de los que 7.175 recibían tratamiento con warfarina. Se analizó a los pacientes que usaron como mínimo un medicamento de prescripción por un periodo mínimo de 7 días concomitante con warfarina. Las interacciones potenciales fueron analizadas usando Epocrates online, Stockley´s Drug Interactions, y bases de datos locales de interacciones.
Resultados: El numero medio de medicamentos usados concomitantemente con warfarina fue de 4,8 (DE=1,9) (hombres 4,7 DE=2,0; mujeres 4,9 DE=2,0). No se encontraron interacciones potenciales en el 38% de los pacientes, se observó una interacción potencial en el 29%, y se encontraron dos o más interacciones potenciales en el 33% de pacientes. El número medio de interacciones potenciales fue de 1,2 por paciente, prácticamente igual en hombres y en mujeres. Las interacciones potenciales estaban asociadas con el número de medicamentos. Se detectaron interacciones relacionadas con la warfarina en el 57% de los pacientes, y el número de interacciones relacionadas con warfarina varió de 1 a 5. Las más frecuentes fueron el uso de warfarina con AINE (14%), seguidas de sinvastatina (9%) y amiodarona (7%).
Conclusión: Este estudio muestra que el 57% de los pacientes ambulatorios que reciben warfarina en estonia tienen medicamentos potencialmente interaccionando con la warfarina en sus esquemas terapéuticos. La mayoría de las interacciones (14%) con warfarina estaban asociadas a los AINE.
Palabras clave: Warfarina. Interacciones de medicamentos. Pacientes Ambulatorios. Estonia.
Introduction
Thromboembolic diseases including stroke and venous thromboembolism are associated with a significant increase in mortality and morbidity.1 These conditions primarily affect older patients with atrial fibrillation, and this group of patients ultimately requires effective and well-tolerated long-term therapy.2,3 Currently, the most commonly used drug for the prevention of thromboembolic diseases is warfarin.4-6 Warfarin plays a significant role in anticoagulant therapy due to the availability of an oral formulation and its low cost and efficacy. Although warfarin has been used in clinical practice for more than 60 years, it is still associated with several adverse reactions/events due to its narrow therapeutic index, patient compliance, high inter-individual variability, and interactions with many other drugs.7-10
Warfarin is one of the top three drugs (alongside insulin and digoxin) that are most commonly associated with severe drug-related complications.11 The main reason for this is the variety of interactions (about 400) with other drugs.12,13 Recent data suggest that warfarin drug interactions might be a major source of the bleeding events in patients receiving anticoagulant treatment.14 Warfarin interactions can vary from theoretical (clinically insignificant) to severe life-threatening interactions. The most frequent clinical manifestation of the interactions is bleeding, which can vary from superficial ecchymoses to severe haemorrhage in the gastrointestinal tract or brain. It was reported that about 70% of warfarin interactions are associated with an increase in its effects, while only 15% are reported to reduce its effects.15
Elderly patients are more commonly affected by the adverse effects of warfarin than younger patients. The incidence rate of major bleeding episodes following warfarin therapy was twice as high in patients aged 65 and older than in younger patients.16 The risk for developing excessive anticoagulation is particularly high in the initial phases of therapy.17 Elderly patients also have a predisposition to receive polypharmacotherapy due to multisystem disease, and they often use warfarin concomitantly with many other drugs.8,18
Therefore, it is important to know which drugs patients are taking concomitantly with warfarin. This should be taken into account by general practitioners since they have the most comprehensive overview of the treatment regimen of the patient.
In Estonia, warfarin is widely prescribed by general practitioners to prevent and treat thromboembolic diseases. To date, there has been no systematic analysis of warfarin complications or the potential risk of its interactions with other drugs in the outpatient population in Estonia. The aim of the study was to analyze the incidence of potential interactions in the prescription schemes in Estonia in a cohort of outpatients receiving warfarin treatment.
Methods
In a retrospective prevalence study, the data of the Estonian Health Insurance Fund concerning prescriptions redeemed in the first 6 months of 2008 were analyzed. The Estonian Health Insurance Fund is the main health insurance provider and covers about 95% of the population of Estonia. All the data acquired from the Estonian Health Insurance Fund was coded. The data included outpatients of both gender aged 50 and older who had received two or more prescriptions of systemic drugs with an intended duration of treatment of a minimum of 7 days during a period of 6 months. The number of patients included in the database was 203,646, comprising 43.1% of the Estonian population aged 50 and over.
Patients who were prescribed only one pharmaceutical product or topically applied drugs were not included in the database. Those patients who had received treatment for less than 7 days were also excluded from the study. The purchase date of the drug was regarded as the start of therapy and the number of defined daily doses (DDDs) determined the duration of therapy.
Patients (n=7,175) who were prescribed warfarin therapy were further identified and analyzed separately. For patients receiving warfarin therapy, the average number of concomitantly used drugs in different age and gender groups was calculated. Potential interactions of the drugs taken were identified using the domestic drug interaction database, KIS (Koostoimete Infosüsteem in Estonian language). This database, developed in Estonia by S. Uibokand and A. Zharkovsky19, is currently being used as an instrument for the detection of potential interactions in prescription schemes in Estonian pharmacies.
The database is organized by potential interactions of drug pairs. The KIS database uses a risk-rating classification of interactions based on previously published criteria13, outlines the possible clinical manifestation of the interaction, and gives recommendations for further actions (e.g. that the concomitant use of a specific drug pair should be avoided or that certain clinical parameters should be monitored).
The data obtained on potential interactions were further verified using two additional interaction databases: Epocrates online20 and Stockley´s Drug Interactions.12 Epocrates online is a database widely used by physicians, and according to current literature21, it demonstrates a high level of validity. To our knowledge, the most comprehensive source of information on drug interactions is Stockley´s handbook, which was therefore chosen as a major source of information.
Potential interactions were taken into account only if present in all three databases. The number of potential interactions in the treatment regimen of each patient was identified.
In cases where a patient underwent several courses of the same drug combination, it was counted only once in this study.
Older patients are likely to receive polypharmacy and therefore, potentially subject to additional interactions, which do not involve warfarin-related interactions and might also have an impact on the development of adverse reactions. As a recent study22 showed, a linear increase in the number of drugs consumed led to an exponential increase in the number of adverse reactions. Therefore, drug interactions, not related to warfarin were also taken into account.
Statistical analysis was performed using SAS software. The mean standard deviation (SD) was calculated and comparisons were made using Tukey´s test. Linear correlation coefficients and Spearman´s correlation coefficients were calculated.
The study was approved by the Ethics Review Committee (ERC) on Human Research of the University of Tartu.
Results
The whole database consisted of 203,646 patients. Over the 6-month inclusion period (Jan-June 2008), these patients received 1,936,961 prescriptions and 698,127 of them were for drugs that were used systemically and concomitantly. A total of 7,175 (3.5%) patients were receiving warfarin therapy, and they were given 34,903 prescriptions.
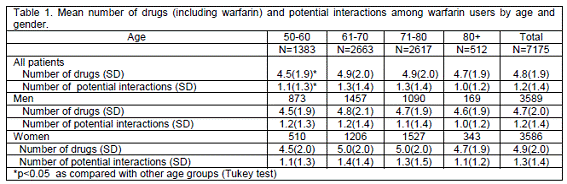
The distribution of the patients receiving warfarin treatment by age and gender is shown in Table 1. The mean number of concomitantly used drugs was 4.8 (SD=1.9), women had a higher mean number drugs: 4.9 (SD=2.0) than men: 4.7 (SD=2.0) (p<0.05). The mean number of potential interactions was 1.2 (SD=1.4) per patient. Women tended to use more drugs concomitantly, and they had slightly (p<0.05) more potential interactions in the drug regimen than men (Table 1).
The number of concomitant drugs increased with the age of the patient, being lowest in the 50-60 age group: 4.5 (SD=1.9) compared with other age groups (Table 1).
No potential interactions in treatment regimens were found in 2,713 patients (38%), and these patients had the lowest number of concomitantly used drugs: 3.6 (SD=1.33) (Table 2). One potential interaction was observed in 29% of patients and two or more potential interactions in 33% of patients (Table 2). The highest number of interactions was 13 in one patient. Over half (57%) of patients had potential interactions, related to warfarin and the number of interactions related to warfarin per patient varied from 1 to 5. Drug interactions not related to warfarin were detected in 5% of patients receiving warfarin treatment. The most common warfarin-related interaction was with non-steroidal anti-inflammatory drugs (NSAIDs) (14% of warfarin-using patients), followed by simvastatin (9%), amiodarone (7%), propafenone (5%), allopurinol (4%), and amitriptyline (4%) (Table 3). The interactions not related to warfarin (5%) included beta-blocking agents and antidiabetic drugs and the combination of NSAIDs with diuretics and ACE inhibitors.
Our study showed a positive correlation between the potential interactions and the number of concomitant drugs (Pearson correlation coefficient 0.985, p<0.0001). Multifactorial analysis did not reveal any additional associations between the number of interactions and gender and age.
Logistic regression analysis showed that, using the patient population of 50-60 years of age as a reference group, the odds of exposure to a potential interaction among warfarin users were the highest in the 61-70 years-old age group [OR=1.20; 95%CI 1.06:1.35]. The odds of exposure were greater in women than in men with an OR=1.21 [95%CI 1.11:1.32].
Discussion
For the first time, the occurrence of potential interactions in the treatment regimens of patients receiving warfarin therapy was studied in Estonia. Our study shows that of 7,175 patients, 57% patients had potential interactions related to warfarin and 5% of patients had interactions not related to warfarin. The number of potential interactions increased in parallel with the number of prescriptions. A strong positive correlation between the number of drugs used and number of potential interactions underscores the need to minimize the number of drugs in the treatment regimen and to avoid drugs with potential interactions.23 Analysis of the literature shows a great variability in the incidence of potential interactions in patients receiving warfarin treatment.
Some studies24,25 have reported a much higher incidence of potential drug interactions in treatment schemes. In a study by Verhovsek et al.24, 79% of 107 long-term care patients receiving warfarin treatment had interacting drugs in their treatment schemes. This study revealed 5 most common warfarin interacting drugs: acetaminophen, citalopram, acetylsalicylic acid, diltiazem and simvastatin. The higher occurrence of potential interactions in that study24 is probably due to the older population of the included patients. Kotirum et al.25 evaluated the frequency of potential interactions in patients treated with warfarin in Thailand from June 1999 to August 2004 and also demonstrated a higher percentage of warfarin-related interactions, which occurred in 83.6% of patients. The higher percentage of potential interactions in that study may be related to the fact that over-the-counter (OTC) medicines (ibuprofen and aspirin) were included. In Estonia, aspirin and ibuprofen (400 mg) are sold without prescription. Therefore, the size of the group of patients in our study, which concomitantly uses warfarin and NSAIDs, is likely underestimated. Also, in the study by Kotirum et al.25, the most frequent interacting drug was paracetamol, which in Estonia is sold without prescription.
Our study shows that, in Estonia, women receiving warfarin are prescribed a higher number of medications: 4.9 (SD=2.0) compared to men: 4.7 (SD=2.0) and at the same time they have a slightly higher number of potential interactions than men. This is consistent with a previous study26, in which female gender and polypharmacy increased the risk of bleeding caused by anticoagulant therapy.
Our analysis shows that 95% of patients who have interacting drugs in the treatment regimen have an increased risk of bleeding. It is important to draw attention to the interaction of NSAIDs and warfarin, which is considered to be one of the most important interactions in clinical practice. In our study, the most frequent possible interaction with warfarin was NSAIDs, which accounted for 14% of patients and 24% of interactions related to warfarin. This drug combination has been reported to increase the risk of bleeding by 2-5 times, in comparison to warfarin alone.26,27 Narum et al. studied 289 bleeding event case reports in Norway and found that in more than 50% of cases, the patients used medicines potentially interacting with warfarin.14 In a study on the prevalence of potentially hazardous interactions among Australian veterans, Roughead et al. found NSAIDs co-dispensing with warfarin in 7.2% cases, which is less than in our study.28 In some studies, the percentage of patients who concomitantly used warfarin and NSAID, was higher in comparison to our study. In the study of Snaith et al.29, the prevalence of combination NSAID and warfarin was 21%. The Thai study by Kotirum et al.25 (1,093 patients treated with warfarin from June 1999 to August 2004) showed that 43% of patients receiving warfarin treatment also used NSAIDs.
Increased risk of bleeding is associated not only with concomitant use of warfarin with NSAIDs, but also with other drugs. Analysis of the treatment regimens in our study shows that a considerable proportion of patients concomitantly used warfarin with simvastatin (9%), amiodarone (7%), propafenone (5%) allopurinol (4%), and levothyroxine (4%). These combinations also might result in an increased risk of bleeding.12,30-36 The literature describing warfarin and simvastatin interactions is limited to a few case reports. Simvastatin is a substrate for CYP3A4 and may potentially interact with warfarin metabolism through competitive inhibition of the enzyme.37,38 A previous study showed that patients co-treated with statins had lower plasma 10-hydroxywarfarin concentrations39, which indicates that they had slower metabolism of (R)-warfarin. The same study, however, failed to demonstrate any significant effect of simavastatin or lovastatin on the clearance of (R)-warfarin.39 Therefore, the impact of statins on the pharmacokinetics of warfarin remains unclear. To avoid a possible mild risk of interaction, simvastiatin could be replaced by atorvastatin, which does not interact with warfarin pharmacokinetics.40 The frequency of the concomitant use of simvastatin with warfarin in other studies24,25 varies from 2.6% to 10%. In our study, the frequency of concomitant use of amiodarone (7%) was also higher than in other studies.24,25 Previous publications demonstrated that amiodarone augments the effects of warfarin via inhibition of warfarin elimination30,32,33,39,41, especially in the initial phase of concomitant use of drugs42,43, and this interaction is considered to be clinically significant.25 In contrast, Hermann et al.39 showed that there were no statistically significant differences in the concentration of 10-hydroxy-warfarin between two groups of patients who received warfarine alone or warfarin with amiodarone. It is important to note that the patients in the study of Herman et al.39 were treated with low doses of amiodarone, whereas the drug interacts with warfarin in a dose-dependent manner.33
Other co-administered drugs among our patients on warfarin treatment were propafenone (5%), allopurinol (4%), levothyroxine (4%), which can also affect warfarin pharmacokinetics and thereby increase the risk of bleeding.34-36 It was shown that co-adminitration of propafenone with warfarin increased warfarin concentration by 38% in healthy volunteers.34 Therefore, warfarin dose must be reduced in this combination. Allopurinol in the usual clinical dosages does not affect the concentration of warfarin.35 Most probably the clinical significance of the interaction between warfarin and allopurinol is low.25 There is little data available on warfarin and levothyroxine interactions. Levothyroxine may inhibit warfarin metabolism or decrease protein binding of the drug36, and therefore this interaction is considered to be clinically significant.25
Our study shows that some patients also used a drug that can induce a reduction of warfarin efficacy. The only such drug in our study, which could decrease the efficacy of warfarin, was carbamazepine, which was used by 1% of warfarin-using patients. Carbamazepine acts as a cytochrome P450 enzyme inducer and may increase the metabolism of warfarin.44 Herman et al.39 found significantly higher clearance of the (S)-and (R)-warfarin when patients used warfarin with carbamazepine. These patients also required higher doses of warfarin for desired anticoagulant effect.39 Kotirum et al. consider the clinical significance of this interaction as potentially high.25
Our study shows that 4.2% of patients on warfarin therapy also received amitriptyline. There are few articles about the co-administration of amitriptyline with an anticoagulant, but some authors have described the fluctuation of the INR values in response to this combination.45 The clinical significance of this interaction is unclear.
Our analysis was based upon prescriptions, and therefore our study method did not address the following questions: 1) the compliance of the patients-whether the patients took the prescribed drugs and whether they followed their treatment regimen; 2) the use of non-prescription drugs. As discussed above, it is not known whether the patients were using acetaminophen for pain relief or low-dose aspirin for antithrombotic prevention, or other non-prescription drugs and herbal medicines; 3) the consumption of alcohol, tobacco and food rich in vitamin K; 4) the INR values of patients and clinically manifested adverse reactions. Not all potential interactions are manifested clinically, depending upon several patient-related factors: age, concomitant diseases, and gender. In order to address the above limitations, the current research is being continued with an analysis of patients´ case report forms. A questionnaire has also been developed concerning the drugs used and clinical interactions. According to our preliminary data, minor bleeding incidents are evident in 15% of cases of warfarin-NSAID co-administration.
Conclusions
The data obtained in this study show that 57% of outpatients in Estonia receiving warfarin are also prescribed drugs potentially interacting with warfarin and 5% of patients on warfarin have other interacting drug combinations in their treatment schemes. A strong correlation was found between the number of concomitantly used drugs and the number of potential interactions, which indicates that practicing doctors do not always consider possible interactions when establishing a treatment regimen for their patients. Most interactions with warfarin are associated with increased risk of bleeding, especially with NSAIDs (14%), followed by simvastatin (9%), amiodarone (7%), propafenone (5%), allopurinol (4%) and levothyroxine (4%). Of potential drugs, that can reduce the efficacy of warfarin, only carbamazepine was found in the included treatment regimens. Our study shows that a considerable number of outpatients receiving warfarin therapy are prescribed interacting drugs, and most of them lead to an increased risk of bleeding. This study aims to draw attention to the importance of acknowledging that drug interactions are very common in ambulatory practice. For patients in whom the use of a combination of interacting drugs cannot be avoided, a careful monitoring of adverse events should be continuously conducted. Patients on warfarin should also be instructed to avoid using over-the-counter medicines by both general practitioners and pharmacists.
Acknowledgements
We thank the Estonian Health Insurance Fund and the State Agency of Medicines for assistance with data collection and Siim Uibokand, Rauno Viin and Joosep Lassmann for statistical analysis of the data. This research was supported by the European Union through the European Regional Development Fund of Estonia and Estonian Research Foundation Grant No. 7955.
Conflict of interest
None to declare.
References
1. Geerts WH, Bergqvist D, Pineo GF, Heit JA, Samama CM, Lassen MR, Colwell CW. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 Suppl):381S-453S. [ Links ]
2. Lip GY, Hart RG, Conway DS. Antithrombotic therapy for atrial fibrillation. BMJ. 2002;325(7371):1022-1025. [ Links ]
3. Lip GY, Agnelli G, Thach AA, Knight E, Rost D, Tangelder MJ. Oral anticoagulation in atrial fibrillation: A pan-European patient survey. Eur J Intern Med. 2007;18(3):202-208. [ Links ]
4. Segal JB, McNamara RL, Miller MR, Kim N, Goodman SN, Powe NR, Robinson KA, Bass EB. Prevention of thromboembolism in atrial fibrillation. A meta-analysis of trials of anticoagulants and antiplatelet drugs. J Gen Intern Med. 2000;15(1):56-67. [ Links ]
5. Hirsh J, Dalen J, Anderson DR, Poller L, Bussey H, Ansell J, Deykin D. Oral Anticoagulants: Mechanism of Action, Clinical Effectiveness, and Optimal Therapeutic Range. Chest. 2001;119(1 Suppl):8S-21S. [ Links ]
6. Johnson JA. Warfarin: an old drug but still interesting. Pharmacotherapy. 2008;28(9):1081-1083. [ Links ]
7. Hanley JP. Warfarin reversal. J Clin Pathol. 2004;57(11):1132-1139. [ Links ]
8. Juurlink DN. Drug interactions with warfarin: what clinicians need to know. CMAJ. 2007;177(4):369-371. [ Links ]
9. Pirmohamed M. Warfarin: almost 60 years old and still causing problems. Br J Clin Pharmacol. 2006;62(5):509-511. [ Links ]
10. Pirmohamed M, James S, Meakin S, Green C, Scott AK, Walley TJ, Farrar K, Park BK, Breckenridge AM. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ. 2004;329(7456):15-19. [ Links ]
11. Budnitz DS, Shehab N, Kegler SR, Richards CL. Medication use leading to emergency department visits for adverse drug events in older adults. Ann Intern Med. 2007;147(11):755-765. [ Links ]
12. Baxter K. Stockley´s Drug Interactions (9 Edition). Pharmaceutical Press. 2010. [ Links ]
13. Bachmann KA. Lexi-Comp´s drug interactions handbook: the new standard for drug and herbal interactions. Lexi-Comp. 2004. [ Links ]
14. Narum S, Solhaug V, Myhr K, Johansen PW, Brørs O, Kringen MK. Warfarin-associated bleeding events and concomitant use of potentially interacting medicines reported to the Norwegian spontaneous reporting system. Br J Clin Pharmacol. 2011;71(2):254-262. [ Links ]
15. Holbrook AM, Pereira JA, Labiris R, McDonald H, Douketis JD, Crowther M, Wells PS. Systematic overview of warfarin and its drug and food interactions. Arch Intern Med. 2005;165(10):1095-1106. [ Links ]
16. Spencer FA, Gore JM, Lessard D, Emery C, Pacifico L, Reed G, Gurwitz JH, Goldberg RJ. Venous thromboembolism in the elderly. A community-based perspective. Thromb Haemost. 2008;100(5):780-788. [ Links ]
17. Hylek EM, Heiman H, Skates SJ, Sheehan MA, Singer DE. Hylek EM, Evans-Molina C, Shea C, Henault LE, Regan S. Major hemorrhage and tolerability of warfarin in the first year of therapy among elderly patients with atrial fibrillation. Circulation. 2007;115(21):2689-2696. [ Links ]
18. Wittkowsky AK, Boccuzzi SJ, Wogen J, Wygant G, Patel P, Hauch O. Frequency of concurrent use of warfarin with potentially interacting drugs. Pharmacotherapy. 2004;24(12):1668-1674. [ Links ]
19. Uibokand S, Zarkovski A. A system for analysis and report of drug interactions. Patent No.091803734-1225. European Patent Office 10.05.10. [ Links ]
20. https://online.epocrates.com (accessed May 8, 2011) [ Links ]
21. Clauson KA, Polen HH, Marsh WA Clinical decision support tools: performance of personal digital assistant versus online drug information databases. Pharmacotherapy. 2007;27(12):1651-1658. [ Links ]
22. Ray S, Pramanik J, Bhattacharyya M, Todi S. Prospective observational evaluation of incidences and implications of drug-drug interactions induced adverse drug reactions in critically ill patients. Indian J Pharm Sci. 2010;72(6):787-792. [ Links ]
23. Johnell K, Klarin I. The relationship between number of drugs and potential drug-drug interactions in the elderly: a study of over 600,000 elderly patients from the Swedish Prescribed Drug Register. Drug Saf. 2007;30(10):911-918. [ Links ]
24. Verhovsek M, Motlagh B, Crowther MA, Kennedy C, Dolovich L, Campbell G, Wang L, Papaioannou A. Quality of anticoagulation and use of warfarin-interacting medications in long-term care: A chart review. BMC Geriatr. 2008;8:13. [ Links ]
25. Kotirum S, Chaiyakunapruk N, Jampachaisri K, Wattanasombat S, Rojnuckarin P. Utilization review of concomitant use of potentially interacting drugs in Thai patients using warfarin therapy. Pharmacoepidemiol Drug Saf. 2007;16(2):216-222. [ Links ]
26. Palareti G, Cosmi B. Bleeding with anticoagulation therapy-who is at risk, and how best to identify such patients. Thromb Haemost. 2009;102(2):268-278. [ Links ]
27. Blann AD, Fitzmaurice DA, Lip GY. Anticoagulation in hospitals and general practice. BMJ. 2003;326(7381):153-156. [ Links ]
28. Roughead EE, Kalisch LM, Barratt JD, Gilbert AL. Prevalence of potentially hazardous drug interactions amongst Australian veterans. Br J Clin Pharmacol. 2010;70(2):252-257. [ Links ]
29. Snaith A, Pugh L, Simpson CR, McLay JS. The potential for interaction between warfarin and coprescribed medication: a retrospective study in primary care. Am J Cardiovasc Drugs. 2008;8(3):207-212. [ Links ]
30. Wells PS, Holbrook AM, Crowther NR, Hirsh J. Interactions of warfarin with drugs and food. Ann Intern Med. 1994;121(9):676-683. [ Links ]
31. Westergren T, Johansson P, Molden E. Probable warfarin-simvastatin interaction. Ann Pharmacother. 2007;41(7):1292-1295. [ Links ]
32. Hamer A, Peter T, Mandel WJ, Scheinman MM, Weiss D. The potentiation of warfarin anticoagulation by amiodarone. Circulation. 1982;65(5):1025-1029. [ Links ]
33. Sanoski CA, Bauman JL. Clinical observations with the amiodarone/warfarin interaction: dosing relationships with long-term therapy. Chest. 2002;121(1):19-23. [ Links ]
34. Kates RE, Yee YG, Kirsten EB. Interaction between warfarin and propafenone in healthy volunteer subjects. Clin Pharmacol Ther. 1987;42(3):305-311. [ Links ]
35. Rawlins MD, Smith SE. Influence of allopurinol on drug metabolism in man. Br J Pharmacol. 1973;48(4):693-698. [ Links ]
36. Chute JP, Ryan CP, Sladek G, Shakir KM. Exacerbation of warfarin-induced anticoagulation by hyperthyroidism. Endocr Pract. 1997;3(2):77-79. [ Links ]
37. Bellosta S, Paoletti R, Corsini A. Safety of statins: focus on clinical pharmacokinetics and drug interactions. Circulation. 2004;109(23 Suppl 1):III50-III57. [ Links ]
38. Andrus MR. Oral anticoagulant drug interactions with statins: case report of fluvastatin and review of the literature. Pharmacotherapy. 2004;24(2):285-290. [ Links ]
39. Herman D, Locatelli I, Grabnar I, Peternel P, Stegnar M, Lainscak M, Mrhar A, Breskvar K, Dolzan V. The influence of co-treatment with carbamazepine, amiodarone and statins on warfarin metabolism and maintenance dose. Eur J Clin Pharmacol. 2006;62(4):291-296. [ Links ]
40. Stern R, Abel R, Gibson GL, Besserer J. Atorvastatin does not alter the anticoagulant activity of warfarin. J Clin Pharmacol. 1997;37(11):1062-1064. [ Links ]
41. Almog S, Shafran N, Halkin H, Weiss P, Farfel Z, Martinowitz U, Bank H. Mechanism of warfarin potentiation by amiodarone: dose--and concentration--dependent inhibition of warfarin elimination. Eur J Clin Pharmacol. 1985;28(3):257-261. [ Links ]
42. Lu Y, Won KA, Nelson BJ, Qi D, Rausch DJ, Asinger RW. Characteristics of the amiodarone-warfarin interaction during long-term follow-up. Am J Health Syst Pharm. 2008;65(10):947-952. [ Links ]
43. Heimark LD, Wienkers L, Kunze K, Gibaldi M, Eddy AC, Trager WF, O'Reilly RA, Goulart DA. The mechanism of the interaction between amiodarone and warfarin in humans. Clin Pharmacol Ther. 1992;51(4):398-407. [ Links ]
44. http://medicine.iupui.edu/clinpharm/ddis/table.asp (accessed Nov 8, 2011) [ Links ]
45. Hampel H, Berger C, Kuss HJ, Müller-Spahn F. Unstable anticoagulation in the course of amitriptyline treatment. Pharmacopsychiatry. 1996;29(1):33-37. [ Links ]
46. Wiedermann C, Stockner I. Warfarin-induced bleeding complications -clinical presentation and therapeutic options. Thromb Res. 2008;122(Suppl 2):S13-S18. [ Links ]
47. Wysowski DK, Nourjah P, Swartz L. Bleeding complications with warfarin use: a prevalent adverse effect resulting in regulatory action. Arch Intern Med. 2007;167(13):1414-1419. [ Links ]
48. Sabbe JR, Sims PJ, Sims MH. Tramadol-warfarin interaction. Pharmacotherapy. 1998;18(4):871-873. [ Links ]
49. Hedenmalm K, Lindh JD, Säwe J, Rane A. Increased liability of tramadol-warfarin interaction in individuals with mutations in the cytochrome P450 2D6 gene. Eur J Clin Pharmacol. 2004;60(5):369-372. [ Links ]
50. Penning-van Beest FJ, van Meegen E, Rosendaal FR, Stricker BH. Drug interactions as a cause of overanticoagulation on phenprocoumon or acenocoumarol predominantly concern antibacterial drugs. Clin Pharmacol Ther. 2001;69(6):451-457. [ Links ]
51. Cheetham TC, Levy G, Niu F, Bixler F. Gastrointestinal safety of nonsteroidal antiinflammatory drugs and selective cyclooxygenase-2 inhibitors in patients on warfarin. Ann Pharmacother. 2009;43(11):1765-1773. [ Links ]
52. Malhi H, Atac B, Daly AK, Gupta S. Warfarin and celecoxib interaction in the setting of cytochrome P450 (CYP2C9) polymorphism with bleeding complication. Postgrad Med J. 2004;80(940):107-109. [ Links ]
53. Israel DS, Stotka J, Rock W, Sintek CD, Kamada AK, Klein C, Swaim WR, Pluhar RE, Toscano JP, Lettieri JT, Heller AH, Polk RE. Effect of ciprofloxacin on the pharmacokinetics and pharmacodynamics of warfarin. Clin Infect Dis. 1996;22(2):251-256. [ Links ]
54. Schelleman H, Bilker WB, Brensinger CM, Han X, Kimmel SE, Hennessy S. Warfarin with fluoroquinolones, sulfonamides, or azole antifungals: interactions and the risk of hospitalization for gastrointestinal bleeding. Clin Pharmacol Ther. 2008;84(5):581-588. [ Links ]
55. Angaran DM, Dias VC, Arom KV, Northrup WF, Kersten TG, Lindsay WG, Nicoloff DM. The comparative influence of prophylactic antibiotics on the Ann Surg. 1987;206(2):155-161. [ Links ]
56. Pea F, Furlanut M. Pharmacokinetic aspects of treating infections in the intensive care unit: focus on drug interactions. Clin Pharmacokinet. 2001;40(11):833-868. [ Links ]
57. Ng HJ, Crowther MA. Azathioprine and inhibition of the anticoagulant effect of warfarin: evidence from a case report and a literature review. Am J Geriatr Pharmacother. 2006;4(1):75-77. [ Links ]
58. Whyte IM, Buckley NA, Reith DM, Goodhew I, Seldon M, Dawson AH. Acetaminophen causes an increased International Normalized Ratio by reducing functional factor VII. Ther Drug Monit. 2000;22(6):742-748. [ Links ]
59. Hylek EM, Heiman H, Skates SJ, Sheehan MA, Singer DE. Acetaminophen and other risk factors for excessive warfarin anticoagulation. JAMA. 1998;279(9):657-662. [ Links ]
60. Meeks ML, Mahaffey KW, Katz MD. Danazol increases the anticoagulant effect of warfarin. Ann Pharmacother. 1992;26(5):641-642. [ Links ]
61. O'Reilly RA. The stereoselective interaction of warfarin and metronidazole in man. N Engl J Med. 1976;295(7):354-357. [ Links ]
62. Kim KY, Mancano MA. Fenofibrate potentiates warfarin effects. Ann Pharmacother. 2003;37(2):212-215. [ Links ]
63. de Teresa E, Vera A, Ortigosa J, Pulpon LA, Arus AP, de Artaza M. Interaction between anticoagulants and contraceptives: an unsuspected finding. Br Med J. 1979;2(6200):1260-1261. [ Links ]
64. Zingone MM, Guirguis AB, Airee A, Cobb D. Probable drug interaction between warfarin and hormonal contraceptives. Ann Pharmacother. 2009;43(12):2096-2102. [ Links ]
Received (first version): 02.06.11
Accepted: 17.01.12