Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Pharmacy Practice (Granada)
versión On-line ISSN 1886-3655versión impresa ISSN 1885-642X
Pharmacy Pract (Granada) vol.11 no.1 Redondela ene./mar. 2013
ORIGINAL RESEARCH
Demonstration of anticoagulation patient self-testing feasibility at an Indian Health Service facility: A case series analysis
Demostración de la factibilidad del autocontrol por pacientes de la anticoagulación en un centro del servicio sanitario indio: análisis de serie de casos
Ryan R. Schupbach1, John M. Bousum2 and Michael J. Miller3
1PharmD, BCPS, CACP. Clinical Assistant Professor. Department of Pharmacy, Clinical and Administrative Sciences, College of Pharmacy, The University of Oklahoma Health Sciences Center. Tulsa, OK (United States)
2PharmD. Clinical Pharmacist. Claremore Comprehensive Health Care Facility. Claremore, OK (United States)
3RPh, DrPH, FAPhA. Associate Professor. Department of Pharmacy: Clinical and Administrative Sciences, College of Pharmacy, The University of Oklahoma Health Sciences Center. Tulsa, OK (United States).
ABSTRACT
Background: Anticoagulation patient self-testing (PST) represents an alternative approach to warfarin monitoring by enabling patients to use coagulometers to test their international normalized ratio (INR) values. PST offers several advantages that potentially improve warfarin management.
Objective: To describe implementation and associated performance of a PST demonstration program at an Indian Health Service (IHS) facility.
Methods: A non-consecutive case series analysis of patients from a pharmacy-managed PST demonstration program was performed at an IHS facility in Oklahoma between July 2008 and February 2009.
Results: Mean time in therapeutic range (TTR) for the seven patients showed a small, absolute increase during the twelve weeks of PST compared to the twelve weeks prior to PST. Four of the seven patients had an increase in TTR during the twelve week course of PST compared to their baseline TTR. Three of four patients with increased TTR in the final eight week period of PST achieved a TTR of 100%. Of the three patients who experienced a decrease in TTR after initiating self-testing, two initially presented with a TTR of 100% prior to PST and one patient had a TTR of 100% for the final eight weeks of PST. The two patients not achieving a TTR of 100% during the twelve week PST period demonstrated an increase in TTR following the first four weeks of PST.
Conclusion: Although anticoagulation guidelines now emphasize patient self-management (PSM) only, optimal PST remains an integral process in PSM delivery. In the patients studied, the results of this analysis suggest that PST at the IHS facility provided a convenient, alternative method for management of chronic warfarin therapy for qualified patients. More than half of the patients demonstrated improvement in TTR. Although there is a learning curve immediately following PST initiation, the mean TTR for the entire PST period increased modestly when compared to the time period prior to PST.
Key words: Self Care; Anticoagulants; Warfarin; International Normalized Ratio; Drug Monitoring; United States.
RESUMEN
Antecedentes: La automedición por el paciente (PST) de la anticoagulación representa un abordaje alternativo a la monitorización de warfarina al capacitar a los pacientes a utilizar coagulómetros para medir los valores de su ratio internacional normalizado (RIN). La PST ofrece varias ventajas que mejoran el manejo de warfarina.
Objetivo: describir la implantación y la actuación asociada a un programa de demostración de PST en un centro del Servicio Sanitario Indio.
Métodos: Se realizó un análisis de una serie de casos no consecutivos de un programa de PST realizado por una farmacia en un Servicio Sanitario Indio de Oklahoma desde julio 2008 a Febrero 2009.
Resultados: El tiempo medio en rango terapéutico (TRT) para los siete pacientes mostró un pequeño incremento absoluto durante los 12 meses de PST comparado con los 12 meses previos al PST. Cuatro de los siete pacientes tuvieron un aumento de TRT durante las 12 semanas de tratamiento comparado con el TRT al inicio. Tres de los cuatro con aumento de TRT, alcanzaron un TRT del 100% al final del periodo de 8 semanas. De los tres pacientes que tuvieron un descenso de TRT después de inicial la automedición, dos presentaban un TRT antes del PST del 100% y un paciente tenía un TRT del 100% al final de las 8 semanas de PST. Los dos pacientes que no alcanzaron un TRT del 100% durante las 12 semanas de PST demostraron un aumento de TRT después de las 4 semanas iniciales.
Conclusión: Aunque las guias de anticoagulación actualmente solo enfatizan el auto-manejo por el paciente, el PST optimo es parte integral de ese auto-manejo En los pacientes estudiados, los resultados de este análisis sugieren que el PST en un centro del Servicio Sanitario Indio proporciona un método conveniente y alternativo para el manejo crónico del tratamiento con warfarina en pacientes cualificados. Más de la mitad de los pacientes demostró un aumento del TRT. Aunque existe una curva de aprendizaje inmediatamente después de la iniciación del PST, la media de TRT para el periodo completo de PST aumentó modestamente comparada con el tiempo anterior al PST.
Palabras clave: Auto-cuidado; Anticoagulantes; Warfarina; Relación Normalizada Internacional; Monitoreo de medicamentos; Estados Unidos.
Introduction
Oral anticoagulant therapy with warfarin has been shown to reduce the risk of thromboembolic events in patients with atrial fibrillation1-5 and mechanical heart valves.6-9 The safety and efficacy of warfarin is based on maintaining the patient´s international normalized ratio (INR) within specific therapeutic ranges which are individualized for each patient. Management can be difficult due to warfarin´s narrow therapeutic window, variability in dose responses among patients, numerous drug and diet interactions, and lack of laboratory standardization.10 Close monitoring of the INR is required to ensure efficacy in the prevention of thromboembolic events and safety against serious bleeding or hemorrhage. Time in therapeutic range (TTR) is generally recognized as a measurement of the quality of anticoagulation management and reflects the amount of time the INR value is maintained within the patient´s therapeutic range. An inverse relationship between TTR and warfarin-related adverse events exists and is well established in the aforementioned warfarin patient cohorts.11-13 Because American Indian and Alaskan Native adults are 2.3 times more likely to be diagnosed with diabetes predisposing them to increased risk of stroke, optimizing TTR is essential to anticoagulation management.14 Optimal management of warfarin therapy reduces unnecessary visits to healthcare providers for routine INR assessment and dosage adjustment.
INR patient self-testing (PST) is an alternative approach to warfarin monitoring by enabling patients to use coagulometers to test their INR values in the convenience of their home setting. PST requires the capacity to comprehend and apply new and complex technical skills. Therefore cognitive barriers and limited health literacy may restrict eligibility for inclusion in PST programs. PST offers several advantages that may potentially improve warfarin management including increased frequency of INR monitoring potentially leading to improved anticoagulation outcomes; greater patient convenience; enhanced patient involvement in disease management; and better patient understanding of diet and activity-related warfarin interactions.10
A systematic review of fourteen studies involving PST revealed significant reductions in thromboembolic events, all-cause mortality, and major hemorrhage.15 In a randomized, prospective trial, patients who self-tested had a statistically significant increase in TTR compared to patients monitored in an anticoagulation clinic (93% versus 75%; p=0.003). The authors noted the TTR for the self-testing group improved after the initial four weeks, increasing from 90% in weeks 1 through 4 to 100% during weeks 4 through 8.16 Another study assessed PST versus warfarin management in an anticoagulation clinic and reported a statistically significant increase in TTR from 57% in the 6 months immediately prior to the intervention compared to 71% during the 6 month study period in the self-testing group.17 No significant differences in bleeding or thromboembolic events were reported in either study.16,17 A study conducted through the Department of Veterans Affairs, The Home INR Study (THINRS), reported that PST decreased events such as stroke, major bleed, and death compared to patients undergoing routine management through an anticoagulation clinic, but that differences were not statistically significant. The THINRS trial did however demonstrate modest yet significant improvements in patient satisfaction with anticoagulation therapy and quality of life.18
In 2001, Centers for Medicare and Medicaid Services (CMS) issued a national coverage memorandum for home INR monitoring through PST for mechanical heart valve patients treated chronically with warfarin. To improve warfarin-related outcomes in these high-risk patients, CMS reported that these patients should require more frequent testing due to the many factors that affect the bioavailability of warfarin.19 Weekly INR testing in patients with mechanical heart valves has been shown to improve anticoagulation management by greater maintenance of INR values within the target range.20 In March 2008, CMS expanded coverage to include patients receiving chronic warfarin therapy for atrial fibrillation and prevention of recurrent venous thromboembolism.21 At the time of this study, the 2008 American College of Chest Physicians (ACCP) guidelines for antithrombotic and thrombolytic therapy recognized both PST and patient self-management (PSM). Patient self-testing is a prerequisite step in PSM, which requires the patient to not only test but also adjust their anticoagulant dosages accordingly. Both PST and PSM were considered effective alternatives to conventional INR management in the 2008 guidelines for patients who are "suitably selected and trained".10 The more recent 2012 ACCP guidelines suggest PSM only, which includes PST for motivated patients who demonstrate competency in self-management strategies.22
The following patient characteristics have been recognized to define optimal eligibility for PST: willingness to participate; functional (cognitive and physical) capacity to perform self-testing; reliability to comply with standard PST policies and procedures; communicability by telephone; and the presence of a caregiver who meets the listed criteria.23 For PST reimbursement, CMS requires that patients: be anticoagulated with warfarin for at least three months prior to initiating self-testing; receive face-to-face educational programs regarding anticoagulation therapy and proper use of the coagulometer; be assessed for continued correct use of the coagulometer; and test no more frequently than once per week.21 Of note, INR testing can occur more frequently than once weekly if necessary, however, there is no reimbursement for additional testing.
Given the ACCP´s recommendation for PST in suitable patients at the time of this study, a qualifying group of patients was identified and selected to demonstrate initial feasibility of PST within an Indian Health Service (IHS) facility. This report represents the first known evaluation of a PST program in the IHS and serves to inform the future direction and feasibility of PSM programs.
Methods
Design
A non-consecutive case series analysis of patients from a pharmacy-managed PST program demonstration between July 2008 and February 2009 was performed at an IHS facility in Oklahoma. The Anticoagulation Clinic (ACC) at the facility, a federal hospital in the IHS, routinely manages anticoagulation therapy for approximately 180 American Indian & Alaskan Native patients. The INR results for consenting patients enrolled in the PST program were described and plotted for twelve week periods before and after patient self-testing was initiated. The study protocol received expedited approval from the Cherokee Nation and IHS Oklahoma City Area institutional review boards (IRBs) prior to program initiation. The IRB from The University of Oklahoma Health Sciences Center also reviewed and approved the project.
Patient Sample
During July 2008, ACC patient´s electronic health records (EHR) were reviewed by the investigators (RS, JB) for initial consideration of PST enrollment. Patients with mechanical prosthetic heart valves in the aortic or mitral position were identified for inclusion in the initial PST demonstration given CMS' prior approval of PST testing in this patient subgroup. All patients enrolled in the program had an INR target range of 2.5 - 3.5. These patients were further screened to ensure they had received anticoagulation therapy for at least three months in accordance with CMS requirements for PST. Patients were selected through an informal assessment process for program inclusion based on a final review of additional favorable patient factors including: functional capacity of patient or care giver to perform PST determined subjectively by the ACC staff; willingness and voluntary consent to participate in the PST demonstration program; and availability of telephone communication for contact. Final approval of eligibility for potential PST patients was determined by each patient´s primary care physician. Physicians prescribed the PST coagulometer and testing strips for weekly self-testing. Informed consent was obtained from all patients participating in the PST demonstration program. Patients were excluded if they received anticoagulation therapy for indications other than mechanical heart valve replacement.
Implementation
Patients enrolled in the PST program received a CoaguChek XS® coagulometer consistent with the device used in the ACC. An initial, individual training session was performed by an ACC pharmacist. On both initial ACC enrollment visits and on all subsequent visits, patients received comprehensive warfarin counseling and education (i.e., drug-drug, drug-food interactions). During patient selection, all PST patients were required to have had received warfarin for at least 3 months which mirrored the 2008 CMS decision memorandum on home INR monitoring requirements (i.e., "beneficiary has been anticoagulated for least three months prior to use of the home INR device"). All coagulometers and related testing supplies were provided by an independent diagnostic training facility (IDTF) and paid for by the patient´s insurer.
Initially, the coagulometer and testing supplies were mailed directly to the ACC for patient training by ACC staff. Subsequent testing supplies were then mailed directly to the patient´s home. Initial training sessions with an ACC pharmacist were scheduled for one-hour periods with additional time allotted for patients when necessary as determined by the pharmacist. Family members were strongly encouraged to attend the training sessions. During the initial training sessions, each patient or pre-identified care giver was trained on use of the coagulometer and then asked to demonstrate proper technique using the teach-back method. Patients or care givers repeated the required steps until the technique was deemed appropriate by the ACC pharmacist. Patients were also provided a step-by-step instruction sheet customized by the ACC detailing proper use of the coagulometer. Pharmacists reassessed technique during follow-up visits in the ACC.
Measurements
Patient background characteristics included age, sex, heart valve type, duration of warfarin therapy, concomitant antiplatelet therapy, diagnosis of type 2 diabetes mellitus, and cognitive impairment (as determined by a diagnosis in the patient´s EHR of any known disorder affecting mental cognition). Diagnosis of diabetes was selected as a characteristic for review due to the potential for greater understanding and use of portable testing devices and fingerstick testing in diabetic patients.
Single INR values were reported by each patient once weekly via telephone to a proprietary databank provided by the IDTF for reporting and storage of INR values. Patients were instructed to self-test and immediately report INR values each Tuesday morning to encourage routine, habitual weekly self-testing while also considering ACC staff availability. After patients reported their INR results, the INR values for each individual were immediately faxed to the ACC by the IDTF for pharmacist interpretation. All INR results were recorded manually in the patient´s EHR and in the facility´s ACC anticoagulation management database. The database calculated cumulative and individual TTR by linear interpolation through a modified Rosendaal methodology.24 Three monthly (i.e., four week intervals) TTR values were recorded for the twelve week periods before and after self-testing was initiated.
Prior to the initiation of PST, the decision was made to contact patients by telephone for the initial four PST weeks after each INR value was reported to assess and verify the reported value, determine if changes in warfarin therapy were warranted, and inquire about any patient issues involving procedure or use of the coagulometer. PST program patients maintained monthly face-to-face follow-up visits in the ACC during the study period. During these visits, patients were required to present with their coagulometers to demonstrate proper fingerstick technique and use of the device while also allowing the ACC staff to verify correct INR reporting by the patients as well as proper device maintenance and storage in the patients´ home.
Analysis
Descriptive statistics were used to profile the aggregate characteristics of PST program participants as well as the time in therapeutic range for the observation period. Given the case series design, the mean TTRs during each observation period were described. TTR during each observation period before and after initiation of PST was plotted for each study participant to profile anticoagulation performance over time. Plots were reviewed to identify trends in anticoagulation control before and after initiation of the PST program.
Results
Nine patients were initially identified and selected during August of 2008 for enrollment into the PST program. One patient was removed from study inclusion because the patient´s primary physician determined that the patient was unsuitable for self-testing due to diminished cognitive ability. The remaining eight patients were enrolled and underwent individual, face-to-face training sessions. Following initiation of the PST training sessions, one patient was removed from the study due to noncompliance with testing and was not included in this analysis. This patient was elderly (69 years old), receiving warfarin for a replaced mechanical heart valve in the mitral position, and was routinely non-compliant with self-testing and reporting despite weekly telephone reminders.
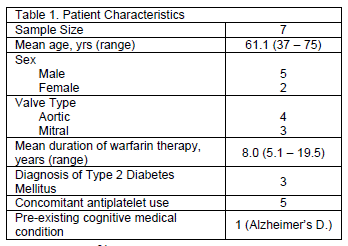
Demographic characteristics for the seven patients are listed in Table 1. Overall, mean TTR showed a small, absolute 3.5% increase during the 12 weeks of PST compared with the 12 weeks prior to PST (Table 2). However, the mean TTR during final 8 weeks of the PST period had an absolute TTR increase of 11.5% (Table 2).
Four of the seven patients had an increase in TTR during the entire twelve week course of PST compared to their baseline TTR (Figure 1). Of note, three of four patients with increased TTR in the final eight week period of PST achieved a TTR of 100%.
Three patients experienced a decrease in TTR after initiating PST. Two of these patients initially presented with a TTR of 100% prior to PST. One of the three patients achieved a TTR of 100% for the final eight weeks of PST. The two patients not achieving a TTR of 100% during the 12 week PST period demonstrated an increase in TTR following the initial decrease during the first four weeks of PST.
Overall, there was an absolute increase in mean TTR of 16.9% from the first four weeks of PST to the final eight weeks of PST. Six of seven patients demonstrated an increase in TTR for the last eight weeks of PST. The only patient who had a decrease in TTR during the final eight weeks of PST compared to the initial four weeks did experience an absolute 7.1% increase in TTR during the entire twelve weeks of PST.
Discussion
In the patients studied, the results of this case series analysis suggest that the PST demonstration program at the IHS facility provided a convenient, alternative method for management of chronic warfarin therapy. More than half of the patients demonstrated improvement in TTR. Although the mean TTR for the entire PST period increased modestly when compared to the time period prior to PST, there appears to be a learning curve immediately following PST initiation. A decrease in TTR often occurred during the first four weeks post initiation of PST. However, during the final eight weeks of PST, 5 of the 7 patients demonstrated improvement in TTR, providing better control of anticoagulation management and reducing risk for bleeding and thromboembolic events. Providers should anticipate transiently suboptimal TTR values immediately following PST training and maintain frequent contact with patients during this crucial period to ensure patient understanding and acceptance of self-testing.
Improper technique or experience with the fingerstick method and coagulometer testing, leading to inaccurate INR results were often reported by patients as primary reasons for reduced TTR during the first four weeks of PST. When patients were consulted by telephone about their INR results, the most common problem reported was the inability to obtain a large enough blood sample. A delay in placing blood on the test strip can lead to inaccurate results.25 Based on data presented in this article, the authors recommend intensive patient contact for the first several weeks following initiation of PST and frequent reassessment of patient´s fingerstick technique to ensure proper sampling.
The majority of patients (6/7) showed an increasing trend in TTR over the final four weeks of the PST observation period. Interestingly, the one patient (MVR3) with a decreased TTR during the final four weeks of PST maintained 100% TTR for the four weeks before and after initiating PST. A chart review of ACC progress notes on follow-up PST visits during the final four weeks revealed that the patient experienced numerous episodes of nausea and vomiting which can affect vitamin K intake as well as absorption of warfarin.10 In this patient, increased variability in the TTR would likely have occurred regardless of PST. With weekly PST, the clinic was able to monitor this patient closely to identify INR fluctuations and ensure no major complications occurred.
Another PST patient (MVR2) had a diagnosis of and was receiving therapy for Alzheimer´s disease. The ACC staff consulted the patient´s physician and collectively determined that with reliable household support and self-awareness of the condition, the patient could be successfully enrolled into the PST program. Also, the patient´s Alzheimer´s status was considered mild by his physician and was managed with a single medication. Practitioners might be tempted to exclude a patient from PST with this diagnosis due the propensity to fail to self-test and/or report INR results. Despite a decline in TTR over the twelve week study period, the patient achieved 94% TTR in the final four week period of PST. After initial follow-up visits during the self-testing period, the patient reported noncompliance with medication recommendations for warfarin. The patient would give verbal feedback on all changes in dosing recommendations, but stated taking different doses. With weekly follow-up, the authors were able to isolate the noncompliance issues and provide appropriate, detailed counseling. In these circumstances, patients must be both physically and mentally capable of using the coagulometer properly and/or have a caregiver trained to operate the coagulometer as noted in the guidelines for implementation of PST.23 This represents a current unmet need in PST selection as no formal assessment of cognitive capacity is specifically defined for PST. Based on the current experience, we do not recommend patients with cognitive deficiencies be followed by self-testing in the absence of capable caregiver assistance. Furthermore, this highlights the need for comprehensive, standardized eligibility criteria for PST inclusion. A local PST screening/eligibility form was developed following the study period merging both literature and clinical experience. Criteria included in the ACC´s PST eligibility form for screening purposes included the aforementioned CMS requirements (i.e., >3 months receiving warfarin; approved indication), interest in self-testing, regular access to a telephone, adequate cognitive and physical capacity abilities (briefly assessed by inquiring with the patient whether they utilize the internet regularly), and a review of the patient´s appointment and medication compliance. Several "value-added" assessment items included whether the patient is diabetic suggesting familiarity with fingersticking technique and whether the patient has adequate family/spousal support.
Concerns related to PST identified in this demonstration project also included patients forgetting to perform weekly tests or report INR results. During telephone communication with patients each week of the first four PST weeks as designated in the protocol, another identified problem reported by patients was remembering to test weekly on the assigned day. Limited understanding of patient´s responsibility to report their INR results immediately after self-testing was also noted despite this issue addressed in the initial PST training. After the initial four weeks of PST, patients rarely reported problems with technique or use of the coagulometer. A majority of the patients were able to demonstrate correct use of the coagulometer at routine ACC follow-up appointments.
Although the THINRS trial18 showed no statistically significant differences in bleeding and thromboembolic events in PST patients, humanistic outcomes including patient satisfaction and quality of life were shown to be significantly improved. A patient satisfaction survey was developed and distributed to all PST patients at the ACC. Despite positive feedback regarding survey results, many surveys were received in an unblinded fashion (i.e., patients writing in names despite being instructed not to do so). The lack of anonymity and small sample size limited our ability to use the results without potentially jeopardizing confidentiality assurances. Therefore, the decision was made to not include the satisfaction survey results in the current report. In the future, the use of a validated patient satisfaction tool (e.g., the Duke Anticoagulation Satisfaction Scale as used in the THINRS trial) is recommended.
There are several limitations to this case-series analysis. For example, the number of patients followed was small and the observation time was short. Although many PST studies have been conducted over a 6 month period, 12 weeks sufficiently reflects a patient´s initial self-testing learning curve. Future studies should monitor the impact of PST over longer time duration to evaluate PST performance sustainability. This study used TTR as a clinically acceptable process indicator for evaluation to demonstrate feasibility. No patients experienced serious adverse events during participation. While this study does not demonstrate the clinical outcome benefit of PST in patients, the improvement in TTR suggests that patients who self-test may experience improved anticoagulation management by virtue of increased frequency of monitoring when compared with coordinated anticoagulation management. Additionally, with current ACCP guidelines now advocating PSM only, self-testing remains only one aspect, albeit integral, to providing this alternative model of care. With feasibility of PST processes firmly established at the IHS facility and emphasis on PSM, the logical next step is implementation of PSM. Furthermore, selecting patients in a nonrandom method could introduce selection bias, however, the selection method used was chosen to mimic actual self-testing patient selection practices that would be applied in routine practice to ensure relevance and easier translation of the findings into practice. Lastly, the reported INR results from patients, at the time of testing, were assumed to be correct. During the PST period, patients presenting to the ACC for monthly visits were required to demonstrate correct testing and use of their coagulometer. The memory function of the coagulometers was reviewed during these visits to ensure patient INR reporting accuracy. No inaccuracies were discovered for any patients.
PST is not feasible for all patients in all health care settings. While there is a need for guidance to facilitate implementation, detailed and complete policies and procedures for eligibility and management of patients who self-test are essential. In order to determine the effect of PST on patient-specific outcomes such as bleeding, thromboembolic events, and mortality, larger studies powered to detect these outcomes are necessary. Although not within the realm of this study, a cost analysis would also helpful to determine potential financial benefits, if any, of a PST program, given the potential to reduce clinic and patient burden of warfarin management.
Conclusions
To effectively implement PSM, PST must be optimized. Patient self-testing was demonstrated to be a viable and effective alternative for managing qualified patients receiving chronic warfarin therapy at the IHS facility. When identifying patients for self-testing, practitioners should consider the willingness to participate; functional (cognitive and physical) capacity to perform self-testing; reliability to comply with standard PST policies and procedures; adequate communication by telephone; and the presence of a caregiver who meets the listed criteria as reported in guidelines for implementation of PST.23 Patience and persistence is required during early periods of PST as TTR may initially decline but ultimately improve, as shown in this study. Given the demonstrated potential, the ACC at the IHS facility is currently expanding its PST program to increase patient enrollment and continues to review PST policy and procedures to refine eligibility criteria and ensure optimal anticoagulation performance. Further analyses are planned to assess long-term efficacy and potential cost-benefit of self-testing.
Conflict of interest
There are no conflicts of interest reported by any of the three authors and no funding, payment or financial support of any type was provided by any outside entities.
Disclaimer: "The opinions expressed in this paper are those of the authors and do not necessarily reflect the views of the Indian Health Service"
References
1. Petersen P, Boysen G, Godtfredsen J, Andersen E, Andersen B. Placebo-controlled, randomized trial of warfarin and aspirin for prevention of thromboembolic complications in chronic atrial fibrillation: the Copenhagen AFASAK study. Lancet. 1989;1(8631):175-9. [ Links ]
2. Boston Area Anticoagulation Trial for Atrial Fibrillation Investigators. The effect of low-dose warfarin on the risk of stroke in patients with nonrheumatic atrial fibrillation. N Engl J Med. 1990;323(22):1505-11. [ Links ]
3. Ezekowitz M, Bridgers S, James K, Carliner N, Colling C, Gornick C, Krause-Steinrauf H, Kurtzke J, Nazarian S, Radford M, Rickles F, Shabetai R, Deykin D. Warfarin in the prevention of stroke associated with nonrheumatic atrial fibrillation: Veterans Affairs Stroke Prevention in Nonrheumatic Atrial Fibrillation Investigators. N Engl J Med. 1992;327(20):1406-12. [ Links ]
4. Stroke Prevention in Atrial Fibrillation Investigators. Stroke Prevention in Atrial Fibrillation Study: final results. Circulation. 1991;84(2):527-39. [ Links ]
5. European Atrial Fibrillation Trial Study Group. Secondary prevention in non-rheumatic atrial fibrillation after transient ischemic attack or minor stroke. Lancet. 1993;342(8882):1255-62. [ Links ]
6. Szekely P. Systemic embolization and anticoagulant prophylaxis in rheumatic heart disease. Br Med J. 1964;1(5392):1209-12. [ Links ]
7. Fleming HA. Anticoagulants in rheumatic heart-disease. Lancet. 1971;2(7722):486. [ Links ]
8. Adams GF, Merrett JD, Hutchinson WM, Pollock AM. Cerebral embolism and mitral stenosis: survival with and without anticoagulants. J Neurol Neurosurg Psychiatry. 1974;37(4):378-83. [ Links ]
9. Silaruks S, Thinkhamrop B, Tantikosum W, Wongvipaporn C, Tatsanavivat P, Klungboonkrong V. A prognostic model for predicting the disappearance of left atrial thrombi among candidates for percutaneous transvenous mitral commissurotomy. J Am Coll Cardiol. 2002;39(5):886-91. [ Links ]
10. Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G. Pharmacology and management of the vitamin k antagonists. Chest. 2008;133(6 Suppl):160S-198S. doi: 10.1378/chest.08-0670. [ Links ]
11. Palareti G, Legnani C, Guazzaloca G, Lelia V, Cosmi B, Lunghi B, Marchetti G, Poli D, Pengo V. Risk factors for highly unstable response to oral anticoagulation: a case control study. Br J Haematol. 2005;129(1):72-8. [ Links ]
12. Forfar JC. Prediction of hemorrhage during long-term oral coumarin anticoagulation by excessive prothrombin ratio. Am Heart J. 1982;103(3):445-6. [ Links ]
13. White HD, Gruber M, Feyzi J, Kaatz S, Tse HF, Husted S, Albers GW. Comparison of outcomes among patients randomized to warfarin therapy according to anticoagulant control: results from SPORTIF III and V. Arch Intern Med. 2007;167(3):239-45. [ Links ]
14. National Diabetes Fact Sheet, 2011. Accessed on December 4, 2012. Available at http://cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. [ Links ]
15. Heneghan C, Alonso-Coella P, Garcia-Alamino JM, Perera R, Meats E, Glasziou P. Self-monitoring of oral anticoagulation: a systematic review and meta-analysis. Lancet. 2006;367(9508):404-11. [ Links ]
16. White RH, McCurdy SA, von Marensdorff H, Woodruff DE Jr, Leftgoff L. Home prothrombin time monitoring after the initiation of warfarin therapy. Ann Intern Med. 1989;111(9):730-7. [ Links ]
17. Khan TI, Kamali F, Kesteven P, Avery P, Wynne H. The value of education and self-monitoring in the management of warfarin therapy in older patients with unstable control of anticoagulation. Br J Haematol. 2004;126(4):557-64. [ Links ]
18. Matchar DB, Jacobson A, Dolor R, Edson R, Uyeda L, Phibbs CS, Vertrees JE, Shih MC, Holodniy M, Lavori P; THINRS Executive Committee and Site Investigators. Effect of Home Testing of International Normalized Ratio on Clinical Events. N Engl J Med. 2010 Oct 21;363(17):1608-20. doi: 10.1056/NEJMoa1002617. [ Links ]
19. Centers for Medicare and Medicaid Services. Decision Memo for Prothrombin Time (INR) Monitor for Home Anticoagulation Management (CAG-00087N) (Memorandum). Decision memo for prothrombin time (INR) monitor for home anticoagulation management. September 18, 2001. [ Links ]
20. Horstkotte D, Piper C, Wiemer M. Optimal frequency of patient monitoring and intensity of oral anticoagulation therapy in valvular heart disease. J Thromb Thrombolysis. 1998;5(Suppl 1-3):19-24. [ Links ]
21. Centers for Medicare and Medicaid Services. Decision Memo for Prothrombin Time (INR) Monitor for Home Anticoagulation Management (CAG-00087R) (Memorandum). Decision memo for prothrombin time (INR) monitor for home anticoagulation management (CAG-00087R). March 19, 2008. [ Links ]
22. Holbrook A, Schulman S, Witt DM, Vandvik PO, Fish J, Kovacs MJ, Svensson PJ, Veenstra DL, Crowther M, Guyatt GH; American College of Chest Physicians. Evidence-based management of anticoagulant therapy. Chest. 2012 Feb;141(2 Suppl):e152S-84S. doi: 10.1378/chest.11-2295. [ Links ]
23. Ansell J, Jacobson A, Levy J, Voller H, Hasenkam JM. Guidelines for implementation of patient self-testing and patient self-management of oral anticoagulation. Int J Cardiol. 2005;99(1):37-45. [ Links ]
24. Rosendaal FR, Cannegieter SC, van der Meer FJM, Briet E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993;69(3):236-9. [ Links ]
25. CoaguChek XS System (package insert). Indianapolis, IN: Roche Diagnostics Corporation; 2006. [ Links ]
Received (first version): 24.07.12
Accepted: 01.03.13