Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Pharmacy Practice (Granada)
versión On-line ISSN 1886-3655versión impresa ISSN 1885-642X
Pharmacy Pract (Granada) vol.13 no.1 Redondela ene./mar. 2015
ORIGINAL RESEARCH
Management for improving patients´ knowledge and understanding about drug allergy
Manejo para mejora del conocimiento y entendimiento de los pacientes sobre alergias a medicamentos
Narumol Jarernsiripornkul1, Nataporn Chaipichit2, Pansu Chumworathayi3, Janet Krska4
1PhD. Division of Clinical Pharmacy, Faculty of Pharmaceutical Sciences, Khon Kaen University. Khon Kaen (Thailand). narumol@kku.ac.th
2MSc. Division of Clinical Pharmacy, Faculty of Pharmaceutical Sciences, Khon Kaen University. Khon Kaen (Thailand). nata_wow@hotmail.com
3MSc. Department of Pharmacy Service, Srinagarind Hospital, Faculty of Medicine, Khon Kaen University. Khon Kaen (Thailand). aguard.apo@hotmail.com
4PhD. Medway School of Pharmacy, The Universities of Greenwich and Kent at Medway. Chatham (United Kingdom). j.krska@kent.ac.uk
ABSTRACT
Background: Drug allergy a serious adverse drug reaction commonly concerned in healthcare practice. Inadequate documentation and communication between health providers, and limited health literacy and knowledge in patients could contribute to the re-occurrence of allergic reactions.
Objective: To evaluate the effectiveness of initiatives aiming to improve patients´ knowledge, understanding and behavior in preventing recurrent drug allergy.
Methods: A before-and-after study was conducted at an 800-bed university teaching hospital, involving patients with a history of drug allergy. Questionnaires, completed at baseline and one month after receiving information were used to compare knowledge and understanding of drug allergy and behaviors in relation to drug allergy cards. Patients in Group 1 received a brochure only, but patients in Group 2 also received a pharmacist counseling intervention in addition to the brochure. Outcomes were evaluated within intervention group and between intervention groups.
Results: The study included 299 (30.4%) and 100 patients (100.0%) in Groups 1 and 2 respectively who completed the baseline questionnaire, of whom 179 (59.8%) and 96 (96.0%) completed the follow-up questionnaire. At baseline, higher educational levels and possession of a drug allergy card were significantly associated with better knowledge about drug allergy. After intervention, Group 2 had significantly greater increases in mean overall knowledge scores than Group 1 (p<0.01) and also greater increases in the proportions self-reporting carrying and presenting drug allergy cards (p<0.05 and p<0.01).
Conclusions: Pharmacist counseling plus brochure may be more effective than brochure alone in promoting patients´ knowledge of drug allergy and drug allergy card importance.
Key words: Drug Hypersensitivity; Patient Medication Knowledge; Patient Education as Topic; Pharmaceutical Services; Thailand.
RESUMEN
Antecedentes: Las alergias a medicamentos son una reacción adversa grave que preocupan habitualmente en los servicios de salud. Inadecuada documentación y comunicación entre profesionales de la salud y una limitada literacía en salud de los pacientes pueden contribuir a la reaparición de reacciones alérgicas.
Objetivo: Evaluar la efectividad de iniciativas que tratan de mejorar el conocimiento, comprensión y comportamiento de los pacientes para prevenir las reacciones alérgicas recurrentes.
Métodos: Se realizó un estudio antes-después en un hospital universitario de 800 camas que incluía pacientes con historial de alergia a medicamentos. Se utilizaron cuestionario administrados al inicio y un mes después de recibir la información para comparar el conocimiento y comprensión de la alergia a medicamentos y los comportamientos en relación a las tarjetas de alergias a medicamentos. Los pacientes del Grupo 1 recibieron solo un folleto, mientras que los pacientes del Grupo 2 recibieron también una intervención de consejo farmacéutico además del folleto. Se evaluaron los resultados en el grupo intervención y entre los grupos.
Resultados: El estudio incluyó a 299 (30,4%) y 100 pacientes (100%) en los Grupos 1 y 2, respectivamente que completaron el cuestionario al inicio, de los que 179 (59,8%) y 96 (96,0%) completaron el cuestionario de seguimiento. Al inicio, los mayores niveles educativos y la posesión de tarjetas de alergias a medicamentos se asociaron significativamente a un mejor conocimiento sobre alergias a medicamentos. Después de la intervención, el Grupo 2 tuvo incrementos significativamente mayores en la media general de puntuaciones de conocimiento que el Grupo 1 (p<0,01) y también mayores incrementos en las proporciones de auto-reporte y de presentación de tarjetas de alergia a medicamentos (p<0,05 y p<0,01).
Conclusiones: El consejo farmacéutico junto con un folleto puede ser más efectivo que el folleto sólo para promover el conocimiento de los pacientes sobre alergia a medicamentos y la importancia de las tarjetas de alergia a medicamentos.
Palabras clave: Hipersensibilidad a Medicamentos; Conocimiento de la Medicación por el Paciente; Educación del Paciente como Asunto; Servicios Farmacéuticos; Tailandia.
Introduction
Drug allergy is an immunologically mediated response to specific agent in a sensitized person.1 The clinical manifestations of drug allergy are restricted to certain syndromes that are specifically accepted as allergic in nature, which may present as mild to life-threatening reactions.2 The estimated incidence of drug allergy was 0.018-4.2 per 1000 hospitalizations3,4, and the estimated mortality related to drug allergy was 0.09 per 1000 hospitalizations (95%CI 0.06, 0.12).4
In Thailand, the occurrence of anaphylaxis in a university hospital increased from 9.16 per 100,000 admitted persons in 1999 to 55.45 per 100,000 admitted persons in 2004, in which 50.0% of identifiable causes were drugs.5 Practices in the Thai healthcare system could potentially contribute to recurrent drug allergy because patients can obtain medications from pharmacies without prescriptions, and there is inadequate documentation and communication regarding drug allergy between health providers.6,7 In addition, medication names are presented in English, and available medication information leaflets are not commonly designed for patient use, being targeted at healthcare professionals. Hence it can be difficult for patients with poor health literacy to recall medication names and understand printed information.8
While a clear and standardized form is important for documentation of drug allergy9,10, empowering both public and healthcare professionals is also necessary for more complete drug allergy documentation.11 The Thai Ministry of Public Health has mandated that drug allergy cards should be provided to patients experiencing serious Adverse Drug Reactions (ADRs) or ADRs which are intolerable or reduce quality of life to prevent future exposure to these drugs. While cards can be useful, their effectiveness in preventing future recurrence depends on patients´ knowledge and understanding of drug allergy. Patients need to carry their drug allergy cards and show them to healthcare professionals at the point of prescribing. Small studies suggest that pharmacists providing counseling to patients about drug allergy and providing basic information about ADRs and their management, together with written information, could result in improved knowledge.12,13 Neither of these studies assessed whether pharmacist counseling was more effective than written information alone or assessed behaviours related to drug allergy. This study, therefore, aimed to evaluate the effectiveness of management in improving knowledge and understanding about drug allergy and behavior-related to drug allergy cards in patients with a history of drug allergy.
Methods
The study was approved by the Khon Kaen University Ethics Committee for Human Research (Institutional Review Board Number: IRB00001189), and conducted at Srinagarind Hospital, an 800-bed university teaching hospital in northeast Thailand. This was a before-and-after study investigating management that involved providing different designs of educational brochures and the addition of pharmacist counseling to a brochure, in order to improve knowledge and understanding about drug allergy and behavior-related to drug allergy card.
Development of educational brochures
Two newly designed brochures, Brochure A and Brochure B, were specially developed for this study and used to educate patients about drug allergy and the importance of drug allergy cards. Both brochures contained similar information explaining about the definitions of drug allergy and side effects, basic observation of drug allergy symptoms, self-management of drug allergy, and how to prevent recurrence. However, the colors used, and information and illustrations of drug allergy differed between brochures. Brochure A used fair color (light-green) as a background and presented information and illustrations of common drug allergy symptoms, for example, maculopapular rash and urticaria. Brochure B used gaudy color (crimson) as a background and presented information and illustration of serious drug allergy symptoms, for example, anaphylactic shock and Stevens-Johnson syndrome. Content was adapted from publications and electronic sources. A dermatologist and a hospital pharmacist, responsible for ADR monitoring, evaluated the clarity, meaningfulness, appropriateness of wording and the format of the brochures. Following this, the brochures were distributed to 20 outpatients to investigate their understanding of the information provided and their preference on brochure design. Patients' comments were then used to revise the final versions of the two brochures used in the study.
Questionnaire Development
The questionnaire was initially developed using an adapted drug hypersensitivity questionnaire developed by the European Network for Drug Allergy (ENDA) and a model for understanding patient attribution of ADRs.14,15 Content validity of the questionnaire was tested by four experts, two internal medicine doctors and two hospital pharmacists, and calculated for Index of Consistency (IOC). The "think aloud" technique, requiring patients to explain how they think or perceive while completing the questionnaires, was performed in five outpatients who had received drug allergy cards at the pharmacy department to assess ease of use and understanding. Comments and suggestions were used to revise the final questionnaire to ensure its appropriateness. Test-retest reliability was then conducted with fifteen eligible patients by completing two copies of the questionnaire two weeks apart.
This paper-based questionnaire consisted of three sections including demographic data and clinical data (7 questions), questions assessing knowledge and understanding of drug allergy and drug allergy cards (5 questions), and questions assessing behavior-related to drug allergy card (2 questions). There were five domains for evaluating patients' knowledge and understanding of drug allergy. Each domain resulted in a score of one, giving a maximum score of five. To attain the maximum score, respondents were required to:
(i) provide the full name of the drug to which they were allergic
(ii) provide at least one drug allergy symptom
(iii) select one or two correct options from six statements relating to management of drug allergy
(iv) provide all correct responses to four "Yes/ No" statements concerning prevention of recurrent drug allergy
(v) provide all correct responses to five "Yes/ No" statements concerning the purpose of the drug allergy card.
The total score was then dichotomized to provide two levels of knowledge and understanding: low to average (score 0 to 3) and good (score 4 or 5).
Patients had to read and completed the questionnaires by themselves, however, for those who had literacy difficulties, care givers were allowed to carry out these procedures according to patients' responses.
Main study procedures
The study involved 1,085 patients participating in two consecutive study phases: Phase 1 recruited patients for self-education (Group 1), who received the brochure only; Phase 2 recruited patients to a pharmacist-counseled group (Group 2), who received the brochure plus pharmacist counseling.
Phase 1: the subjects were 985 patients who had previously been recorded as having a history of drug allergy identified from the pharmacy database or who were recorded with maculopapular rash, anaphylaxis, erythema multiforme, Stevens-Johnson syndrome or toxic epidermal necrolysis in their medical records. Baseline questionnaires were mailed to these patients. Brochures and follow-up questionnaires were then mailed to patients who returned a completed questionnaire one month later. Each patient received only one brochure, which were randomly allocated by simple random sampling method using table of random digits, therefore enabling the effectiveness of different brochure designs to be tested (Brochure A=149 patients and Brochure B=150 patients). Replacement drug allergy cards were provided to patients who indicated they did not have one in their baseline questionnaire response. Both questionnaires were returned to the researcher by mail. This study enabled selection of the most effective brochure for the Phase 2 study. It was conducted between May 1 and July 31, 2009.
Phase 2: subjects were recruited prospectively during a three month period from in-patients who experienced a drug allergy during current hospitalization or were admitted as a result of a drug allergy or out-patients who were screened positively by pharmacists for previous drug allergy during prescription dispensing (Group 2). All patients received baseline questionnaires directly from a pharmacist and this was returned to the pharmacist directly after completion. After completing the baseline questionnaire, a pharmacist reinforced the information about drug allergy that was contained in the brochure, and also: used a standardized diagram to counsel patients about mechanisms, prognosis and complications of drug allergy; emphasized the need to remember the name of the allergic drug and other drugs that could possibly cause the same allergic reactions; advised patients to tell their close family about this drug allergy; and suggested how replacement drug allergy cards could be obtained. This counseling was performed at the outpatient pharmacy department or at inpatient wards depending on the recruitment site and took approximately 15-20 minutes. Drug allergy cards were also provided to patients whose questionnaire responses indicated they did not have one. Follow-up questionnaires were immediately delivered to patients at the study site and they were returned by mail one month after the intervention. This phase was conducted between August 1 and October 10, 2009.
Patients, who did not return the questionnaires within three-week period after sending mails, received telephone and/or postcard reminders twice at three and six weeks. A flowchart of the overall study procedures is shown in Figure 1.
Data Analysis
The data were analyzed using SPSS version 19.0. Relationships between variables were analyzed using Pearson chi- square test or Fisher's exact test where appropriate. The 95% confidence interval or p-value at 0.05 was chosen to accept or reject the null hypothesis.
Independent t-test or Mann-Whitney U test were used to compare pre- and post-test mean total scores between two groups of patients, while Paired t-test or Wilcoxon Signed-rank test were used to compare mean total scores between pre-and post-intervention. McNemar´s test was used for comparing individual knowledge questions and behaviours at baseline and one month later in both groups.
Stepwise logistic regression was used to test for independent variables identified by univariate analyses as being associated with level of knowledge and understanding assessed at baseline using combined data from Group 1 (299 patients) and Group 2 (100 patients).
Results
Questionnaire development
The Index of Consistency (IOC) of the questionnaires was 0.88, which meant that all questions were related to objectives of the study. The Pearson's correlation coefficient was analyzed for test-retest reliability in "knowledge and understanding of drug allergy and drug allergy card´ section, in which the coefficient was 0.75.
Response rate
Group 1: At baseline, 382 questionnaires were returned (response rate 38.8%), of which 299 were fully completed. The complete follow-up questionnaires were returned from 179 respondents (59.9%) who had received Brochure A (89 patients) and Brochure B (90 patients).
Group 2: All 100 baseline questionnaires were returned and the response rate of follow-up questionnaires was 96.0%.
Brochure selection
There were no differences in the demographic characteristics of gender, age, education, total number of drugs used, number of drug allergies, and drug allergy card availability between patients who received the different brochures. Knowledge and behaviors in relation to drug allergy and drug allergy cards between these two sub-groups were not significantly different [all P>0.05) both at baseline (mean total scores Brochure A=3.21 (SD=1.07) and Brochure B=3.33 (SD=1.00)] and follow-up [Brochure A=3.46 (SD=1.04) and Brochure B=3.52 (SD=0.77)]. Patients receiving both Brochures showed significant improvements in mean total score after receiving the intervention (all P<0.05). The frequency of self-reported presenting and carrying drug allergy cards was slightly lower after the intervention, but this was not statistically significant (Table 1). Both brochures were thus able to be used for the Phase 2 study.
Comparing between Managements
Patient characteristics
The general characteristics of patients in Group 1 and Group 2 patients were similar. The majority were female (59.6%) with a high proportion being aged between 41-60 years old (45.5%), the mean age being 48.41 (SD=17.29) years. The most common educational level was secondary school or lower (63.8%) and the majority had a total income less than 10,000 Baht per month (60.5%). However, patients in Group 1 had more drug allergies than those in Group 2 and were more likely to indicate they possessed a drug allergy card. (Table 2).
Knowledge and Understanding of Drug Allergy
At baseline, the mean total score for drug allergy knowledge and understanding between both groups was not significantly different [Group 1=3.27 (SD=1.03), Group 2=3.54 (SD=0.72); p=0.05]. More than 60% of patients in both groups could report the names of the drugs to which they were allergic at baseline, with a higher proportion of those in Group 2 able to do so (Table 3). Penicillins were the most commonly implicated drugs (25.6%), followed by sulfonamides (10.5%) and tetracycline (5.0%). Most patients in both groups were also able to correctly report symptoms of their drug allergy and again more Group 2 patients were able to provide this information. Skin rash was the most frequently reported symptom (27.5%). The majority of patients in both groups selected one of the correct options for managing drug allergy and responded correctly to statements about preventing recurrence, but failed to correctly identify all statements relating to the purpose of the drug allergy card. The majority of respondents in both groups incorrectly gave a positive response to the statement "It could reduce the visit time at healthcare service" (Group 1: 53.4% and Group 2: 76.0%) and "Apart from the recorded drug on the drug allergy card, it prevents allergic reaction to other drugs" (Group 1: 76.1% and Group 2: 86.0%) (Table 3).
After the intervention, both groups of patients were likely to have higher accuracy in almost every area of knowledge and understanding, however, significant differences were only seen in Group 2 patients in their ability to correctly name the drug to which they were allergic and to correctly identify statements on recurrence prevention (P<0.01 and P<0.05, respectively). The purpose of the drug allergy cards remained as the single most common area where patients´ knowledge and understanding of drug allergy was lacking, having shown only marginal increases. The total scores at follow-up in both groups were significantly increased from baseline scores (P<0.01; both groups). Group 2 patients however had significantly higher up total scores at follow-up than Group 1 patients (Table 3).
Behaviors Concerning Drug allergy Cards
Over 80% of patients in both groups at baseline claimed to always carry their drug allergy cards. After the intervention, there was a greater improvement seen in self-reported card carrying behavior in Group 2 compared to Group 1 (92.8% vs. 79.8%; P<0.05). Most patients also claimed they always showed their drug allergy cards or notified staff about their drug allergies when presenting at healthcare services, again with no differences between groups at baseline. After the intervention, Group 2 patients reported higher frequency of self-reported presentation of their drug allergy cards when compared with Group 1 patients (100% vs. 73.1%; P<0.01) (Table 3).
Patients who had two or more drug allergies were significantly more likely to report carrying drug allergy cards than those who had an allergy to only one drug (OR=3.218; 95%CI: 1.139 - 9.091; P=0.027).
Factors Affecting Knowledge and Understanding
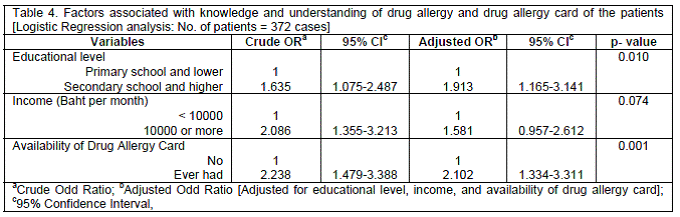
Univariate analysis of all baseline questionnaire responses found that educational level (P<0.001), income (P=0.001), and the availability of a drug allergy card (P=0.021) were significantly associated with good drug allergy knowledge. After adjusting for the independent factors by multivariate analysis, patients who had good baseline scores for knowledge and understanding were those who had graduated from secondary school or higher education (OR=1.913; 95%CI: 1.165 - 3.141; P=0.01), and patients who possessed a drug allergy card (OR=2.102; 95%CI: 1.334 - 3.311; P=0.001) (Table 4).
Discussion
The use of educational brochures containing illustrations of serious drug allergies on gaudy-color background or illustrations of non-serious drug allergies on light-color background were equally effective in improving patients' knowledge and understanding of drug allergy. In addition, pharmacist counseling plus the brochure was found to be more effective than brochure alone in promoting patients´ knowledge and understanding of drug allergies, self-reported card-carrying and presenting cards to health professionals. Therefore, our results suggest that the effort involved in providing important information for patients and also in ensure it is explained is potentially valuable.
The majority of patients (88.2%) in our study were aware that they had experienced allergic reactions to at least one drug. This was a higher rate than that in other studies (17.2%-30.0%)16-18 because our study recruited only patients with a clear history of drug allergy. The finding that 67.7% of all patients were able to name the allergic drugs was also slightly higher than found in a previous study (60.0%).13 Inability to recall drug names could be due to long time intervals since experiencing the allergic reaction in some patients or failure to realize that the drug had caused the allergic reaction19, but could also be due to English drug names being used.
Both interventions, self-education through provision of a brochure alone and pharmacist counseling plus brochure, resulted in a significant increase in knowledge scores, however, a greater improvement in behaviors regarding always carrying and presenting drug allergy cards was found in patients who received pharmacist counseling plus brochure than those who received brochure alone. In addition, higher educational level and owning a drug allergy card were significantly associated with greater knowledge and understanding, which is in line with previous work showing a positive correlation between educational level and knowledge of drug allergy.13 The brochure intervention alone only impacted on overall knowledge score, not on self-reported behaviors. However, the patients in this study self-reported a higher frequency of carrying drug allergy cards than was found in other studies.18,20 Educational materials (e.g. brochures, charts) have previously been found to increase patients' knowledge and understanding of drug allergy, to encourage them in reporting drug allergy history, and discussing their drug-related problems with healthcare professionals.13 The availability of drug allergy cards or bracelets which contain drug names and their allergic symptoms, do help patients to remember and prevent future exposure to the allergic drugs especially when patients are unable to communicate.12 Pharmacists could encourage patients to always carry and present their drug allergy cards during routine service, while intensive counseling at the first exposure of drug allergy may provide better understanding in allergic drugs and other drugs with similar pharmacologic activity, mechanisms of drug allergy, and characteristics of allergic reactions to patients and family members, enabling greater understanding to prevent further occurrences.12,13 Our study suggests that educational brochures should be provided for all drug allergy patients to promote knowledge and understanding of drug allergy, whereas pharmacist counseling should focus on patients with poor knowledge and behaviors related to drug allergy.
There were a number of limitations in the present study. Firstly, the questionnaire required patients to retrospectively complete information about their history of drug allergy. Therefore, especially in Group 1 patients who were recruited from the records on hospital database, might have had difficulties in recalling their allergic experiences. Despite the reminding by telephone and/or postcards were undertaken, recall bias may discourage patients to return mailed questionnaires and have contributed to the low response rate of baseline questionnaires in Phase 1 study (38.8%). Nonetheless, this was similar to the response rate of mailed questionnaires in a previous study involving Thai patients (42.0%).20 This may have resulted in a biased sample, with higher interest and thus knowledge of drug allergy. Secondly, some information required for questionnaire validation, especially physical examinations, may have been recorded incompletely on patients' medical profiles, making assessment of the accuracy of patients´ reports difficult. Thirdly, patients were derived from different populations and were not randomly allocated to groups. Nonetheless, characteristics of patients in both groups did not differ significantly in all key measures with the exception of the availability of drug allergy cards. Finally, although it was instructed that Group 1 patients must read brochure before completing the follow-up questionnaire, their actual compliance in doing so could not be assessed and the observed improvement in mean total knowledge score could not with certainty be as a result of receiving the brochure.
Prospective studies of longer duration should be performed to assess the long term efficacy of both these and other interventions in improving patients´ knowledge, actual behaviors and allergy recurrence rate. In addition, future studies should investigate the cost-effectiveness of brochures in the prevention of recurrent drug allergy and patients´ attitudes towards both brochures and counseling by pharmacists.
Conclusions
Pharmacist counseling with brochures was found to be more effective than brochures alone for improving patients´ knowledge and understanding of drug allergies and drug allergy cards and in promoting drug allergy card carrying behavior. These techniques could be valuable in helping to reduce incidence of recurrent drug allergy.
Acknowledgements
Sincere thanks are expressed to all staff in Pharmacy, Medical records and Statistical Department of Srinagarind Hospital, for their kindness and helpful cooperation.
Conflict of interest
None.
Funding source: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
1. Joint Task Force on Practice Parameters; American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology; Joint Council of Allergy, Asthma and Immunology. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010;105(4):259-273. doi: 10.1016/j.anai.2010.08.002. [ Links ]
2. Executive summary of disease management of drug hypersensitivity: a practice parameter. Joint Task Force on Practice Parameters, the American Academy of Allergy, Asthma and Immunology, the American Academy of Allergy, Asthma and Immunology, and the Joint Council of Allergy, Asthma and Immunology. Ann Allergy Asthma Immunol. 1999;83(6 Pt 3):665-700. [ Links ]
3. Park CS, Kim TB, Kim SL, Kim JY, Yang KA, Bae YJ, Cho YS, Moon HB. use of an electronic medical record system for mandatory reporting of drug hypersensitivity reactions has been shown to improve the management of patients in the university hospital in Korea. Pharmacoepidemiol Drug Saf. 2008;17(9):919-925. doi: 10.1002/pds.1612. [ Links ]
4. Thong BY, Leong KP, Tang CY, Chng HH. Drug allergy in a general hospital: Results of a novel prospective inpatient reporting system. Ann Allergy Asthma Immunol. 2003 Mar;90(3):342-347. [ Links ]
5. Jirapongsananuruk O, Bunsawansong W, Piyaphanee N, Visitsunthorn N, Thongngarm T, Vichyanond P. Features of patients with anaphylaxis admitted to a university hospital. Ann Allergy Asthma Immunol. 2007;98(2):157-162. [ Links ]
6. Sooksriwong C, Leelanitkul S. Perspectives on self-medication practices in Thailand. J Soc Adm Pharm. 2001;18(2):75- 80. [ Links ]
7. Sri-Ngernyuang L. Drug abundance: Situation of drugs and drug distribution in the villages of rural Thailand. Asia Pac J Public Health. 1996-1997;9:18-23. [ Links ]
8. Burapadaja S, Tantipathananandh P, Sirithunyalug B. Consumer´s opinions on reading a medicine Leaflet. Chiang Mai University Journal. 2004;3:155-167. [ Links ]
9. Radford A, Undre S, Alkhamesi NA, Darzi SA. Recording of drug allergies: are we doing enough? J Eval Clin Pract. 2007;13(1):130-137. [ Links ]
10. Gay KJ, Hill C, Bell T. Accuracy of drug-allergy recording in a District General Hospital. Int J Pharm Pract. 2009;17(4):253-235. [ Links ]
11. Khalil H, Leversha A, Khalil V. Drug allergy documentation--time for a change? Int J Clin Pharm. 2011;33(4):610-613. doi: 10.1007/s11096-011-9525-y. [ Links ]
12. Johnson V, Croft C, Crane V. Counseling patients about drug allergies in the inpatient setting. Am J Health Syst Pharm. 2001;58(19):1855-1858. [ Links ]
13. Chaikoolvatana A, Chanakit T, Juengrakpong A. The evaluation of a recurrent Adverse Drug Reaction Prevention Program in the north-east region of Thailand. J Med Assoc Thai. 2006;89(5):699-705. [ Links ]
14. Demoly P, Kropf R, Pichler WJ, Bircher A. Drug hypersensitivity: questionnaire. Allergy. 1999;54(9):999-1003. [ Links ]
15. DeWitt JE, Sorofman BA. A Model for Understanding Patient Attribution of Adverse Drug Reaction Symptoms. Drug Inf J. 1999;33(3):907-920. [ Links ]
16. MacPherson RD, Willcox C, Chow C, Wang A. Anaesthetist's responses to patients' self-reported drug allergies. Br J Anaesth. 2006;97(5):634-639. [ Links ]
17. Valente S, Murray LP. Creative strategies to improve patient safety: allergies and adverse drug reactions. J Nurses Staff Dev. 2011;27(1):E1-5. doi: 10.1097/NND.0b013e31819b5f0b. [ Links ]
18. Hannaford PC. Adverse drug reaction cards carried by patients. Br Med J (Clin Res Ed). 1986;292(6528):1109-1112. [ Links ]
19. Gomes E, Cardoso MF, Praça F, Gomes L, Mariño E, Demoly P. Self-reported drug allergy in a general adult Portuguese population. Clin Exp Allergy. 2004;34(10):1597-1601. [ Links ]
20. Jarernsiripornkul N, Chaisrisawadsuk S, Chaiyakum A, Krska J. Patient self-reporting of potential adverse drug reactions to non-steroidal anti-inflammatory drugs in Thailand. Pharm World Sci. 2009;31(5):559-564. doi: 10.1007/s11096-009-9310-3. [ Links ]
Received (first version):15.09.14
Accepted: 29.01.15