Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Pharmacy Practice (Granada)
versión On-line ISSN 1886-3655versión impresa ISSN 1885-642X
Pharmacy Pract (Granada) vol.14 no.1 Redondela ene./mar. 2016
https://dx.doi.org/10.18549/PharmPract.2016.01.631
ORIGINAL RESEARCH
Adherence to antiretroviral therapy and its determinants among persons living with HIV/AIDS in Bayelsa state, Nigeria
Ismail A. Suleiman1, Andrew Momo2
1 BPharm, MPharm, PhD. Reader, Department of Clinical Pharmacy and Pharmacy Practice. Faculty of Pharmacy, Niger Delta University, Wilberforce Island, Bayelsa State (Nigeria). suleimanismail1@gmail.com
2 BPharm, PharmD. Deputy Director, Pharmacy Department, Federal Medical Centre, Yenagoa, Bayelsa State, (Nigeria). pharmandymomo@yahoo.com
ABSTRACT
Background: A high level of adherence is required to achieve the desired outcomes of antiretroviral therapy. There is paucity of information about adherence to combined antiretroviral therapy in Bayelsa State of southern Nigeria.
Objectives: The objectives of the study were to determine the level of adherence to combined antiretroviral therapy among the patients, evaluate the improvement in their immune status and identify reasons for sub-optimal adherence to therapy.
Methods: The cross-sectional study involved administration of an adapted and pretested questionnaire to 601 consented patients attending the two tertiary health institutions in Bayesla State, Nigeria: The Federal Medical Centre, Yenagoa and the Niger-Delta University Teaching Hospital Okolobiri. The tool was divided into various sections such as socio-demographic data, HIV knowledge and adherence to combined antiretroviral therapy. Information on the patient's CD4+ T cells count was retrieved from their medical records. Adherence was assessed by asking patients to recall their intake of prescribed doses in the last fourteen days and subjects who had 95-100% of the prescribed antiretroviral drugs were considered adherent.
Results: Three hundred and forty eight (57.9%) of the subjects were females and 253 (42.1%) were males. The majority of them, 557 (92.7%) have good knowledge of HIV and combined anti-retroviral therapy with a score of 70.0% and above. A larger proportion of the respondents, 441 (73.4%), had > 95% adherence. Some of the most important reasons giving for missing doses include, “simply forgot” 147 (24.5%), and “wanted to avoid the side-effects of drugs” 33(5.5%). There were remarkable improvements in the immune status of the subjects with an increment in the proportion of the subjects with CD4+ T cells count of greater than 350 cells/mm3 from 33 (5.5%) at therapy initiation to 338 (56.3%) at study period (p<0.0001).
Conclusion: The adherence level of 73.4% was low which calls for intervention and improvement. The combined antiretroviral therapy has significantly improved the immune status of the majority of patients which must be sustained. “Simply forgot” was the most important reason for missing doses.
Key words: Medication Adherence; Antiretroviral Therapy, Highly Active; Epidemiologic Factors; Outcome Assessment (Health Care); Nigeria.
Introduction
Human Immunodeficiency Virus Infection/Acquired Immunodeficiency Syndrome (HIV/AIDS) has become one of the world’s most serious public health challenges1, particularly in developing countries with low per capita income. There are more than 35 million people currently living with HIV/AIDS and nearly 39 million people have died of HIV/AIDS-related causes since the beginning of the epidemic.2 Sub-Sahara Africa which constitutes just 12.0% of the world’s population is home to about 71.0% of people living with HIV/AIDS (PLWHA).3 Nigeria is ranked second after South Africa with an HIV/AIDS population estimate of 3.5 million.4 A national survey also reveals a prevalence of 3.4% in 2012.5
HIV is transmitted primarily via unprotected sexual intercourse, contaminated blood transfusion, contaminated hypodermic needles and from mother to child during pregnancy, delivery, or breastfeeding. However, some body fluids, such as saliva and tears, do not transmit HIV.6 In addition to the health of individuals; HIV/AIDS also have serious impacts on the households, communities as well as economic growth and development of nations.
There is no cure or vaccine for prevention, however, anti-retroviral drugs can slow the course of the disease and may lead to a near-normal life expectancy with optimal adherence to therapy. Nevertheless, HIV/AIDS infection can be prevented or minimized by curbing high-risk behaviours.7
Adherence is defined as “the extent to which a person’s behavior – taking medication following a diet, and/or executing lifestyle changes – corresponds with agreed recommendation from a healthcare provider”.8 For combined antiretroviral therapy (cART) to be effective, a high level of sustained adherence is necessary to suppress viral replication and improve immunological and clinical outcomes. In addition it should decrease the risk of developing anti-retroviral drug resistance, and reduce the risk of transmitting HIV/AIDS.9 It is also well understood and documented that HIV/AIDS requires perfect or near perfect adherence to obtain successful treatment outcome. Recent studies have estimated the required level of adherence for sustained virological suppression to be about 95%.10
Adherence to cART has a number of predictors such as patients variables (age, income, literacy level and social status), type of regimen (type, number of pills, complexity), disease characteristics (stage of HIV, symptoms, opportunistic infections), patient provider relationship (therapeutic relationship, open communication, satisfaction) and clinical setting (accessibility, adherence programme, environment).11,12
Of the various methods of evaluating adherence among HIV/AIDS patients, such as medication refill, self-report using validated questionnaire, visual analogue scale (VAS) and a rating task for 30-day adherence, medication refill adherence was reported to be the strongest predictor of viral suppression.13 Other methods include electronic monitoring, therapeutic drug monitoring, directly observed therapy, heamatologic monitoring and viral load determination.14 Electronic monitoring and unannounced pill counts have been reported to be the best objective measures for real-time assessment of adherence but are of limited value due to cost and issues related to logistics.14,15 Self-report is the most widely used being one of the least tasking and inexpensive particularly suitable for developing countries such as Nigeria with very low per capita income16 and corresponding low per capita health spending. However, there is high probability of over estimation of adherence rate with self-report14 which often necessitates adjunct assessment method(s) of some objective parameters alongside self-report whenever it is used.
Several studies conducted in different countries have reported non-adherence rates ranging from 50% to 80%.17-19 In sub-Saharan Africa, adherence rate varies depending on time and location of studies. One of the studies conducted by Weiser et al, 2003 in Botswana found self-reported and provider assessment adherence rates of 54% and 56% respectively.20 Other studies reported 66% in Uganda21 22% in Cote D’ Ivoire22 and 71% in South Africa.23
In Nigeria, adherence rates from previous studies conducted have ranged from as low as 44% being adherent24 to more than 95%.25 For instance, adherence levels of 49.2% was reported in Port-Harcourt26, 58% in Benin27, 62.9% in Ibadan28, 44% in Ile-Ijesha29, 62.8% in Keffi, north central30, 80% amongst children in Kano31, and 75.3% in Enugu.32
Evidence-based data from developing countries regarding antiretroviral therapy adherence rates, and the effectiveness of support interventions are still limited most especially in Bayelsa State of Nigeria, hence the need for such investigation.
The objectives of the study were to determine the level of adherence of PLWHA to cART, evaluate the improvement in their immune status and identify reasons for sub-optimal adherence to therapy.
Methods
Ethical approval was sought and granted by the Research and Ethics Committee (REC) of the Niger-Delta University Teaching Hospital (NDUTH) and also from the Management of Federal Medical Centre (FMC), Yenagoa. Well informed patients also consented before enrollment into the study.
The study was conducted at Antiretroviral Clinics in two tertiary health institutions in Bayelsa State: FMC, Yenagoa and NDUTH, Okolobiri. These two tertiary hospitals render specialized healthcare services to residents of Bayelsa and neighboring states and have a combined monthly turnover in excess of 5,000 patients. They provide comprehensive antiretroviral services to almost 80% of all the PLWHA in the state.
The study population includes the 1,366 adult PLWHA in FMC and 987 adults PLWHA in NDUTH who are on cART and have commenced treatment before March, 2014. Inclusion criteria were: Confirmed HIV/AIDS patients aged 15 years or older who had started cART three months prior to the study and are willing and able to participate. Patients who were critically ill and those with psychiatric disorders were excluded. Appropriate sample size was calculated based on the total number of enrolled patients which was a minimum of 310 patients from FMC and 285 patients from NDUTH.
The cross-sectional study which lasted two months involved administration of an adapted and pretested questionnaire to consecutive patients in the months of July and August 2014 by trained research assistants. These patients were well informed about the study and met the inclusion criteria. Each of the patients was interviewed for about 25-30 minutes by the trained research assistants on clinic days predominantly of Wednesdays.
The Adult AIDS Clinical Trial Group (AACTG) follow up Questionnaires for screening adherence and barriers to adherence33 was the adapted tool, with slight modifications. The tool was divided into various sections such as socio-demographic data, psychosocial history, HIV knowledge and adherence to antiretroviral drug.
Information about the CD4+ T cells count was obtained from the patient’s medical records.
Adherence was assessed by asking patients to recall their intake of prescribed doses in the last fourteen (14) days. In an attempt to minimize recall bias, patients were asked about their adherence over the previous day, previous 3 days and previous week up to the last 14 days preceding the study. The degree of Adherence to the drugs over the last 14 days was estimated using the following formula28;

From the formula, level of adherence by individual patient was classified into those with less than 95% adherence and those with 95-100% adherence. Subjects who took 95-100% of the prescribed antiretroviral drugs were considered adherent.
Data generated from the questionnaire was sorted, coded and analyzed using Statistical Package for Social Sciences (SPSS) and GraphPad Prism for Windows Instat Version 3 (GraphPad Software San Diego, CA, USA). Descriptive statistics were used in the presentation of results. Data in percentages were further analysed using Chi Square test or Fisher’s exact. At 95% confidence interval, a 2-tailed p-value less than 0.05 was considered significant.
Results
A total of six hundred and one (601) eligible adult PLWHA made up of 313 from FMC, Yenagoa and 288 from NDUTH were interviewed. Three hundred and forty eight (348; 57.9%) were females and 253 (42.1%) were males. The largest proportion, 314 (52.2%), of those interviewed were in the age group of 26-35 years followed by 151 (25.1%) in the 36-45 years age group. Forty Four (7.3%) are below 25 years, while 92 (15.3%) were above 46 years of age. More than half of the sampled population (326; 54.2%) was married, 147 (24.5%) were single, the remaining 128 (21.3%) are divorced, widowed or co-habiting. About one–third of the respondents, 211 (35.1%), had a senior secondary school education, another one-third (208; 34.6%) had tertiary education while the remaining 182(30.3%) had primary education or none at all. More than half of the respondents, 336 (55.9%) were self-employed, 162(27.0) were full time employee, the remaining 103 (17.2%) were student, unemployed or retired. There was significant association between male and female for all the variables (See Table 1 for details).
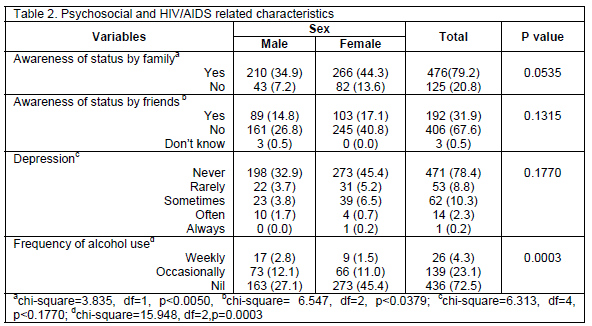
A large proportion, 476 (79.2%), of the respondent’s family were aware of their HIV status while one-third, 192 (31.9%), of the respondent’s friends knew about their HIV status as well. Majority of the study population, 471 (78.4%), never felt depressed in the last four (4) weeks preceding the study while 129 (21.5%) were depressed to varying degrees. The results also showed that about one-quarter of the subjects 65 (27.4) had ingested alcohol (Table 2).
Regarding overall knowledge of HIV/AIDS and treatment, 557 (92.7%) scored at least 70% indicating good knowledge of HIV/AIDS and it’s therapy implications while the remaining 44 (7.3%) were poorly informed. Most of the respondents, 354 (58.9%), were on twice daily triple combination first line regimen (zidovudine/ lamivudine/nevirapine), while 149 (24.8%) were on the once daily triple combination alternative first line regimen (tenofovir/lamivudine/efavirenz) and 31 (5.1%) of them were on the second line lopinavir/ritonavir based regimen. The remaining 67 (11.2%) were either on zidovudine/lamivudine/efavirenz (52; 8.7%) or tenofovir/lamivudine/nevirapine (15; 2.5%) combinations. Almost two-third of the patients, 349 (58.1%), have been on cART for over 24 months, 130 (21.7%) for 12-23 months while the remaining one-fifth (122; 20.2%) had a treatment duration of 3-11 months. Results also showed that about half of the respondents, 303 (50.4%), did not miss any doses of their cART fourteen days (2 weeks) preceding their interview for the study, while 288 (49.6%) missed at least one dose. One hundred and fifty four (25.6%) missed one dose, 86 (14.3%), missed two doses, the remaining 58 (8.7%) missed three or more doses.
The highest adherence rate of 78.2% was achieved with tenofovir/lamivudine/lopinavir/ boosted with ritonavir regimen (second line two tablets twice daily fixed dose combination) which was use in 23(3.8%) of the subjects. Majority of the patients (354; 58.9%) were however on zidovudine/lamivudine/nevirapine regimen (first line one tablet twice daily fixed dose combination) of which 269 (76.0%) were adherent. The second most frequently utilized combination was tenofovir/lamivudine/ efavirenz (alternative first line one tablet once daily fixed dose combination (149; 24.8%) with adherence proportion of 115 (77.2%). The other three combinations were associated with high degree of non-adherence rate ranging from 46.0% for zidovudine/lamivudine/efavirenz and 53.0% for tenofovir/lamivudine/ nevirapine (Table 3).
A larger proportion of the respondents, 441 (73.4%), had =95% adherence to cART, and were classified as adherent. One hundred and eighty-nine (31.4%) of the respondents reported never missing any doses of their medication since they started, while 412 (68.6%) had missed some dose(s) since they started. Adherence rate was slightly higher among FMC subjects (75.7%) with a never missed a dose proportion of 32 .3% than their NDUTH counterparts with 70.8% adherence rate and never missed a dose proportion of 30.2%. There was marked improvement in the immune status (CD4+ T cells counts) of the subjects from the time when the cART was initiated as compared to the study period which was statistically significantly different (p<0.0001). Severe immune-suppression in 55.2% of the subjects at therapy initiation was reduced to 14.0% at study period. There was a greater improvement in the proportion of subjects with CD4 cells count with >350 cells from 20 (3.3%) at therapy initiation to 239 (39.8%) at study period in adherent category which implied a 36.5% increment. The poorly adherent category had an increment of 14.3% (13 (2.2%) at therapy initiation to 99 (16.5%)) at study period (Table 4).
Some reasons giving for missing doses of their medications included, “simply forgot” 147 (24.5), “too busy with other things” 43 (7.2%), “felt better” 28 (4.7%), “wanted to avoid the side-effects of drugs” 33(5.5%) (Table 5). Of the twelve variables studied, nine were found to be significantly associated with adherence among the subjects. These include sex (p=0.0196), age (p=0.0001), marital status (p=0.0007), level of education (p=0.0001), occupation (p=0.0470), depression in the past four weeks, HIV/ART related knowledge (0.0001), cART regimen (p=0.0010) and therapy duration (p=0.0016).
Discussion
About three-quarters of the respondents reported an acceptable level of adherence (95% or over) within the fourteen days preceding the interview, while the remaining one-quarter were non-adherent. Similar anti-retroviral adherence rate have been reported in many parts of Nigeria which range from 70.8% to 78.9%.32,34,35
Higher levels of antiretroviral adherence of 80.0% to 86.0% were reported from similar studies in other regions of Nigeria such 80% in Kano.31 However, some studies across the entire country have equally reported lower adherence rates of 44%- 65% to antiretroviral therapy.27-30,32 and even as low as 36.3% among patients with a depressive disorder.35 For all the studies, patient self-report was the mode of assessment. In addition, one study included pill counting27 and another one added focused group discussion.29 Recall period used were mostly 30-day24,27,31,32 and seven-day.28,29 Other studies also adopted recall period ranging from four-day13,33, seven-day11, and 30-day.13 A meta- analysis found that patients in Africa had better adherence than some of their North-American counterparts with pooled estimates of 77% of participants achieving adherence in the Africa Studies.36
The various levels of adherence observed in different location may be due to various reasons such as true differences, or the fact that there is no gold standard in the measurement of this parameter. For example, different studies used different recall period to determine adherence and some set the adherence level of 90%or over as optimum as against 95% or over in this study. Due to this variation in measurement methods of adherence, it is often difficult to compare findings from different studies.
The prevalence of non-adherence (26.6%) in this study is similar to the 26.7% reported by Bello et al.34 in 2011 and others.32,35 It is noteworthy that only 31.4% of the subjects reported 100% adherence which implied that they have never missed any dose of drug since they started cART. There were more females (56.1%) in this category than males (43.9%). Possible reason could be that the females understood the importance of adherence better than the males. More so, the females in the study were much more educated and equally had better scores on HIV and antiretroviral therapy related knowledge ratings. This observation is in line with studies that reported low educational level as a predictor of non-adherence.37 The higher level of adherence associated with literacy level and knowledge of HIV/ART in this study and awareness of the consequences of non-adherence is consistent with previous reports.38
The marked improvement in the immune status (CD4+ T cells) of patients between when cART was initiated and the study period (with about 40.0% reduction in proportion of patient who was severely immuno-suppressed) indicates achievement of desirable outcomes which must be sustained. It also confirms the fact that adherence to cART was appreciable and all efforts by the various stakeholders at ensuring progressive quality of life improvement among PLWHA and reducing the incidence and prevalence must be strengthened.
The fact that “simply forgot” was the main reason for missing doses in majority of the subjects indicates the benefit of a reminder program be it electronic or human. It also underscores the need for a supportive environment and why stigmatization should be avoided to gain the much needed empathy and sincere communal psychosocial support. Reminder tools such as mobile alarm, watch, and support from family members have been reported to improved adherence.11 "Too busy to remember" which was ranked second also point to the much needed encouragement in places of work. These reasons are similar to what have been previously cited by several studies as the main risk factors for suboptimal adherence.28,30,35
Missing of doses resulting from “wanting to avoid side effects” which was the third most common reason indicate the urgent need for improved patient education and enlightenment. The minor seeming worsening of patient’s quality of life with antiretroviral drugs is by far preferred to the debilitating conditions of well-being in full blown HIV/AIDS patients, which must be avoided at all costs. Studies have shown that side effects of drugs have consistently been associated with decreased adherence and patients may also self-adjust their regimens because of “side-effects”, “toxicity” or “personal belief”.30,35,39 Side effects of drugs may be unavoidable but can be cushioned if the patient is aware of likely ones before drug administration. Awareness of possible side-effects of administered drugs by patients normally aids their adherence to therapy.39
A low proportion of subjects deliberately missed drug doses because they "felt better". This is a sign of misinformation or carelessness probably due to improved health status. Regular assessment and supporting intervention to promote antiretroviral therapy adherence should be maintained throughout the course of therapy.
Missing of doses by “running out of drugs” as reported by some subjects did not signify shortage of drug or stock-out at the facility level but could be because of “missing appointment dates” for drug collection.
“Fear of others noticing” is real because of stigma in this environment and its overwhelming effects. The citing of “fasting” as a reason for missing doses by some subjects is probably due to their spiritual beliefs. However, Habib et al., reported in a study on adherence to cART during Muslims Ramadan fasting that adherence to cART was similar among fasting and non-fasting patients.40 It is imperative to identify fasting subjects and advise them on what to do regarding administration of their medication during a fast, or place them appropriately on cART regimen that will not undermine their beliefs in spiritual well-being which can impact their global quality of life indices positively as well. Missing of doses resulting from “lack of transport fare to health care facility” is a major concern and underscores the reason while patients should be gainfully employed. It is equally unfortunate that despite extension of ART services to different regions, some persons living with HIV/AIDS were unwilling to seek treatment at the nearest health facility because of stigmatization41 thereby increasing their transportation cost burden. The response of missed doses from “too many drugs to take” reinforced results from other studies that have reported HIV medication as an extremely complicated process that requires numerous doses of medication.33 Studies have also shown that the higher the pill burden, the lower the adherence.8 A feeling of depression which affected adherence negatively was similar to previous reports in which there was antiretroviral adherence level as low as 36.3% among patients with depressive disorder.35
The strength of the study include the adaptation of previously validated instrument, comparative nature between two tertiary hospitals, the collection of information on the immune status (CD4+ T cells) of subjects in addition to self- reported adherence. Collection of information on knowledge of prevention and transmission of HIV/AIDS was also a major strength.
Some of the weaknesses and limitations are the difficulty in subject randomization and the use of all consented consecutive patients, self-report nature of the study and the relatively small sample size though representative. Other weaknesses include lack of facility for viral load determination, one point cross sectional survey as against longitudinal study type, as well as involvement of only two centers in Niger Delta region means there is the need to exercise cautions in results generalization. In addition, administration of drugs relative to meal or dietary instruction was not investigated. Likelihood of error due to difficulty in recall is also a limitation which may affect accuracy. The recall bias was minimized by using graded enquiry from one, three, seven days up to day 14. In addition, recall over a fourteen day period is likely to be more accurate than 30 days and probably more realistic for extrapolation relative to shorter periods of recall.
The collection of data on immune status with statistically significant improvement and knowledge of HIV/AIDS prevention and transmission complemented self-report with good correlation. Also the sample size was calculated to be sure it exceeded the minimum and more than one-quarter of the entire enrollee were actually interviewed.
Suggested further study includes measurement of quality of life of patients alongside CD4+ T cells counts, viral loads and adherence to antiretroviral therapy.
It has been recommended that policies on adherence to drug therapy are put in place that would ensure uniform research, improve teaching, enhanced information, education and communication strategies at the communities as well as hospitals.42
Conclusions
The adherence level among the subjects was 73.4% which implied the proportion of patients who were able to achieve 95% or over anti-retroviral therapy adherence. Out of this, only 31.4% achieved 100% adherence. The adherence pattern also correlates well with improvement in their immune status. Forgetfulness topped the reasons for non-adherence followed by too busy to remember and wanting to avoid side effects. To achieve the desired quality of life improvements for individual subjects and reduce prevalence in the society, concerted efforts are needed to promote adherence to therapy. Strategies to ensure adequate economic and psychosocial support for PLWHA and to reduce stigmatizations should be put in place.
Acknowledgements
The cooperation and support of the staff and management of the hospitals is appreciated.
Conflict of interest
There is no conflict of interest associated with this work.
References
1. Sepkowitz KA. AIDS-the first 20 years. N Engl J Med. 2001;344(23):1764-1772. [ Links ]
2. World Health Organization. Global Health Observatory (GHO) data HIV/AIDS 2015 http://www.who.int/gho/hiv/en/ (accessed on January 9, 2016). [ Links ]
3. Joint United Nations Programme on HIV/AIDS (UNAIDS). Report on the Global AIDS Epidemic; 2013 World Population Data Sheet. The Joint United Nations Programme on HIV/AIDS; 2013. [ Links ]
4. NACA, 2012. National Agency for the Control of AIDS (NACA). Global AIDS Response: Country Progress Report: National Agency for the Control of AIDS, Abuja, Nigeria GARPR 2012. [ Links ]
5. The National HIV/AIDS and Reproductive Health Survey-plus. The Federal Ministry of Health, Nigeria 2012, Abuja, Nigeria. [ Links ]
6. Centers for Disease Control and Prevention (CDC). “HIV and its Transmission” 2003. Centers for Disease Control and Prevention, Atlanta, GA 30329-4027 USA. Available at: http://www.cdc.gov/hiv/basics/transmission.html (accessed on February 26, 2015). [ Links ]
7. Centers for Disease Control and Prevention (CDC). HIV/AIDS surveillance report, 2001;13(2):145. [ Links ]
8. World Health Organization. Adherence to long-term therapies. Evidence for action 2003. www.who.int/chroniccondition/adherencereport/en/ (accessed on February 20, 2014). [ Links ]
9. Murphy DA, Roberts KJ, Martin DJ, Marelich W, Hoffman D. Barriers to antiretroviral adherence among HIV-infected adults. AIDS Patient Care STDS. 2000;14(1):47-58. [ Links ]
10. Chesney MA. The elusive gold standard. Future perspectives for HIV adherence assessment and intervention. J Acquir Immune Defic Syndr. 2006;43(Suppl 1):S149-S155. [ Links ]
11. Shigdel R, Klouman E, Bhandari A, Ahmed LA. Factors associated with adherence to antiretroviral therapy in HIV-infected patients in Kathmandu District, Nepal. HIV AIDS (Auckl). 2014;6:109-116. doi: 10.2147/HIV.S55816 [ Links ]
12. Machtinger EL, Bangsberg DR. Adherence to HIV Antiretroviral Therapy. 2005. Available from: http://hivinsite.ucsf.edu/InSite?page=kb-00&doc=kb-03-02-09 (accessed Jan 12, 2016). [ Links ]
13. Chkhartishvili N, Rukhadze N, Svanidze M,Sharvadze L, Dehovitz JA, Tsertsvadze T, McNutt L, del Rio C. Evaluation of multiple measures of antiretroviral adherence in the Eastern European country of Georgia. J Int AIDS Soc. 2014;17:18885. doi: 10.7448/IAS.17.1.18885. [ Links ]
14. New York State Department of Health AIDS Institute. Pharmacists: Partners in Health Care for HIV-Infected Patients 2006 http://www.hivguidelines.org/clinical-guidelines/hiv-and-pharmacy/pharmacists-partners-in-health-care-for-hiv-infected-patients/ (accessed January 5, 2016). [ Links ]
15. Bangsberg DR. Preventing HIV antiretroviral resistance through better monitoring of treatment adherence. J Infect Dis. 2008;197(Suppl 3):S272-S278. doi: 10.1086/533415. [ Links ]
16. World Bank. World Bank GDP per capita 2014. http://www.data.worldbank.org/indicator/NY.GPD.PCAP.CD (accessed September 6, 2014). [ Links ]
17. Reynolds NR, Testa MA, Marc LG, Chesney MA, Neidig JL, Smith SR, Vella S, Robbins GK; Protocol Teams of ACTG 384, ACTG 731 and A5031s. Factors influencing medication adherence beliefs and self-efficacy in person’s naïve to antiretroviral therapy. A multicentre, cross-sectional study: AIDS Behav. 2004 Jun;8(2):141-150. [ Links ]
18. Amico KR, Toro-Alfonso J, Fisher JD. An empirical test of the information, motivation and behavioural skills model of anti-retroviral therapy adherence. AIDS Care. 2005 Aug;17(6):661-673. [ Links ]
19. Remien RH, Bastos FI, Jnr VT, Raxach JC, Pinto RM, Parker RG, Berkman A, Hacker MA. Adherence to antiretroviral therapy in a context of universal access in Rio de Janeiro, Brazil: AIDS Care. 2007;19(6):740-748. [ Links ]
20. Weiser S, Wolfe W, Bangsberg D. Barriers to antiretroviral Adherence to patients living with HIV infection and AIDS in Botswana. J Acquir Immune Defic Syndr. 2003;34(3):281-288. [ Links ]
21. Byakika-Tusiime J, Oyugi JH, Tumwikirize WA, Katabira ET, Mugyenyi PN, Bangsberg DR. Adherence to HIV antiretroviral therapy in HIV+ Ugandan patients purchasing therapy. Int J STD AIDS. 2005;16(1):38-41. [ Links ]
22. Eholié SP, Tanon A, Polneau S, Ouiminga M, Djadji A, Kangah-Koffi C, Diakité N, Anglaret X, Kakou A, Bissagnené E. Field adherence to highly active antiretroviral therapy in HIV-infected adults in Abidjan, Côte d'Ivoire. J Acquir Immune Defic Syndr. 2007;45(3):355-358. [ Links ]
23. Chabikuli NO, Datonye DO, Nachega J, Ansong D. Adherence to Antiretroviral Therapy, Virologic Failure and Workload at the Rustemburg Provincial Hospital. S Afr Fam Pract (2004) 2010;52(4):350-355. [ Links ]
24. Mohammed MD. Adherence to antiretroviral drugs in North Central Zone of Nigeria. East Centr Afr J Pharm Sci. 2004;7(3):52-55. [ Links ]
25. Iliyasu Z, Kabir M, Abubakar IS, Babashani M, Zubair ZA. Compliance to antiretroviral therapy among AIDS patients in Aminu Kano Teaching Hospital, Kano, Nigeria. Niger J Med. 2005;14(3):290-294. [ Links ]
26. Nwauche CA, Erhabor O, Ejele OA, Akani CI. Adherence to antiretroviral therapy among HIV-infected subjects in a resource limited setting in Niger-Delta of Nigeria. Afr J Health Sci. 2006;13(3-4):13-17. [ Links ]
27. Erah PO, Arute JE. Adherence of HIV/AIDS Patients to Antiretroviral Therapy in a Tertiary Health Facility in Benin City. Afr J Pharmacy Pharmacol. 2008;2:145-152. [ Links ]
28. Olowookere SA, Fatiregun AA, Akinyemi JO, Bamgboye AE, Osagbemi GK. Prevalence and determinants of nonadherence to highly active antiretroviral therapy among people living with HIV/AIDS in Ibadan, Nigeria. J Infect Dev Ctries. 2008;2(5):369-372. [ Links ]
29. Afolabi MO, Ijadunola KT, Fatusi AO, Olasode OA. Determinants of Adherence to Antiretroviral Drugs among People Living with HIV/AIDS in the Ife-ijesha Zone of Osun State, Nigeria. Afr J Prim. Healthcare Fem Med. 2009;1(1):1-6. doi: 10.4102/phcfm.v1i1.6. [ Links ]
30. Pennap GR, Abdullahi U, Bako IA. Adherence to highly active antiretroviral therapy and its challenges in people living with human immunodeficiency virus (HIV) infection in Keffi, Nigeria. J AIDS HIV Res. 2013;5(2):52-58. [ Links ]
31. Mukhtar-Yola M, Adeleke S, Gwarzo D, Ladan ZF. Preliminary investigation of adherence to antiretroviral therapy among children in Aminu Kano Teaching Hospital, Nigeria. Afr J AIDS Res. 2006;5(2):141-144. doi: 10.2989/16085900609490374. [ Links ]
32. Uzochukwu BS, Onwujekwe OE, Onoka AC, Okoli C, Uguru NP, Chukwuogo OI. Determinants of non-adherence to subsidized anti-retroviral treatment in southeast Nigeria. Health Policy Plan. 2009;24(3):189-196. doi: 10.1093/heapol/czp006. [ Links ]
33. Chesney MA, Ickovics JR, Chambers DB, Gifford AL, Neidig J, Zwickl B, Wu AW. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. Patient Care Committee & Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG). AIDS Care. 2000;12(3):255-266. [ Links ]
34. Bello SI. HIV/AIDS patients’ adherence to antiretroviral therapy in Sobi Specialist hospital, Ilorin, Nigeria. Glob J Med Res. 2011;11:16-25. [ Links ]
35. Olisah VO, Baiyewu O, Sheikh TL. Adherence to highly active antiretroviral therapy in depressed patients with HIV/AIDS attending a Nigerian University hospital clinic. Afr J Psychiatry (Johannesbg). 2010;13(4):275-279. [ Links ]
36. Mills EJ, Nachega JB, Buchan I, Orbinski J, Attaran A, Singh S, Rachlis B, Wu P, Cooper C, Thabane L, Wilson K, Guyatt GH, Bangsberg DR. Adherence to antiretroviral therapy in sub-Saharan Africa and North America: A meta-analysis JAMA. 2006;296(6):679-690. [ Links ]
37. Nemes MI, Carvalho HB, Souza MF. Antiretroviral therapy adherence in Brazil. AIDS. 2004 Jun;18(Suppl 3):S15-S20. [ Links ]
38. Fisher JD, Amico KR, Fisher WA, Cornman DH, Shuper PA, Trayling C, Redding C, Barta W, Lemieux AF, Altice FL, Dieckhaus K, Friedland G; LifeWindows Team. Computer-based intervention in HIV clinical care setting improves antiretroviral adherence: the LifeWindows Project. AIDS Behav. 2011;15(8):1635-1646. doi: 10.1007/s10461-011-9926-x. [ Links ]
39. Groh K, Audet CM, Baptista A, Sidat M, Vergara A, Vermund SH, Moon TD. Barriers to antiretroviral therapy adherence in rural Mozambique. BMC Public Health. 2011;11:650. doi: 10.1186/1471-2458-11-650. [ Links ]
40. Habib AG, Shepherd JC, Eng MK, Babashani M, Jumare J, Yakubu U, Gebi UI, Saad M, Ibrahim H, Blatter WA. Adherence to antiretroviral therapy (ART) during Muslin Ramadan Fasting. AIDS Behav. 2009;13(1):42-45. doi: 10.1007/s10461-008-9412-2. [ Links ]
41. Wasti SP, van Teijlingen E, Simkhada P, Randall J, Baxter S, Kirkpatrick P, Gc VS. Factors influencing adherence to antiretroviral treatment in Asian developing countries: a systematic review. Trop Med Int Health. 2012;17(1):71-81. doi: 10.1111/j.1365-3156.2011.02888.x [ Links ]
42. Rickles NM, Brown TA, McGivney MS, Snyder ME, White KA. Adherence: a review of education, research, practice, and policy in the United States. Pharm Pract (Granada) 2010;8(1):1-17. [ Links ]
Received (first version): 26.06.15
Accepted: 12.02.16