Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Pharmacy Practice (Granada)
versión On-line ISSN 1886-3655versión impresa ISSN 1885-642X
Pharmacy Pract (Granada) vol.14 no.1 Redondela ene./mar. 2016
https://dx.doi.org/10.18549/PharmPract.2016.01.677
ORIGINAL RESEARCH
Inclusion of salt form on prescription medication labeling as a source of patient confusion: a pilot study
Dana J. Mcdougall1, James D. Hoehns2, Tara T. Feller3, Savana J. Kriener4, Matthew J. Witry5
1 PharmD, BCPS. Covenant Cancer Treatment Center, Waterloo, IA (United States). At time of writing: Northeast Iowa Medical Education Foundation. Dana.Mcdougall@wfhc.org
2 PharmD, BCPS, FCCP. Northeast Iowa Medical Education Foundation, Waterloo, IA; & Department of Pharmacy Practice and Science, College of Pharmacy, University of Iowa. Iowa City, IA (United States). jhoehns@neimef.org
3 PharmD, MPH. Health-System Pharmacy Administration Resident, The John Hopkins Hospital. Baltimore, MD (United States). At time of writing: College of Pharmacy, University of Iowa. tara.feller13@gmail.com
4 PharmD. Sterling Drug. Cresco, IA (United States). At time of writing: College of Pharmacy, University of Iowa. novak.savana@gmail.com
5 PharmD, PhD. Department of Pharmacy Practice and Science, College of Pharmacy, University of Iowa. Iowa City, IA (United States). matthew-witry@uiowa.edu
Funding: This study was supported by the John E. Sutherland Clinical Research Center, Northeast Iowa Medical Education Foundation, Waterloo, Iowa.
ABSTRACT
Background: It has been estimated that 10,000 patient injuries occur in the US annually due to confusion involving drug names. An unexplored source of patient misunderstandings may be medication salt forms.
Objective: The objective of this study was to assess patient knowledge and comprehension regarding the salt forms of medications as a potential source of medication errors.
Methods: A 12 item questionnaire which assessed patient knowledge of medication names on prescription labels was administered to a convenience sample of patients presenting to a family practice clinic. Descriptive statistics were calculated and multivariate analyses were performed.
Results: There were 308 responses. Overall, 41% of patients agreed they find their medication names confusing. Participants correctly answered to salt form questions between 12.1% and 56.9% of the time. Taking more prescription medications and higher education level were positively associated with providing more correct answers to 3 medication salt form knowledge questions, while age was negatively associated.
Conclusions: Patient misconceptions about medication salt forms are common. These findings support recommendations to standardize the inclusion or exclusion of salt forms. Increasing patient education is another possible approach to reducing confusion.
Key words: Medication Errors; Patient Safety; Drug Labeling; Drug Packaging; Pharmaceutical Preparations; Health Literacy; United States.
Introduction
Medication errors based on drug labeling continues to be a problem in the United States. According to the United States Pharmacopeia/Institute for Safe Medication Practices (USP/ISMP) Medication Error Reporting Program, at least one-quarter of reported errors involve look-alike and sound-alike drug names.1 It has been estimated that 10,000 patient injuries occur in the US annually because of patient, prescriber, and pharmacist confusion involving drug names.2 Labeling related errors include, but are not limited to, orthographic similarity (ex. hydroxyzine vs. hydralazine), phonetic similarity (ex. Zantac® vs. Zyrtec®), and simple differences between letters and numbers (capital letter “I” vs. the number 1 in some fonts). Several methods have been developed in order to decrease the errors which occur from name confusion. Proposed methods include using tallman letters3 (buPROPion vs. busPIRone) and contrasting different letters4 (hydroxyzinee vs. hydralazine).
An issue that has not been investigated is whether patient confusion over medication names occurs when the salt of the active ingredient is included on the prescription vial or monograph. In a letter to the editor, McDougall et al., reported two cases of patient confusion and subsequent medication non-adherence resulting from the inclusion of the salt form of the active ingredient on a prescription vial or monograph.5 In one instance, a fully cognitive patient discontinued taking a prescribed potassium supplement after receiving a prescription for losartan potassium. The patient was under the impression that the potassium salt form of losartan provided therapeutic potassium supplementation. In a separate case, another patient experienced confusion between HCl (hydrochloride) and HCTZ (hydrochlorothiazide). She had been prescribed both benazepril 20 mg once daily and hydrochlorothiazide 25 mg once daily. However, her benazepril prescription label read “benazepril HCl 20 mg” and she incorrectly thought that she was receiving a combination tablet of her two medications. Therefore, on her own accord she discontinued her hydrochlorothiazide.
It is estimated that 50% of medications are administered as salts.1 Manufacturers often combine an active drug with a salt in order to produce better biopharmaceutical properties.6 An analysis of the U.S. Food and Drug Administration (FDA) Approved Drug Products with Therapeutic Equivalence Evaluations (Orange Book) database reported that 37.9% of oral active pharmaceutical ingredients existed as a salt with basic properties (e.g. chloride, bromide, pamoate anions), while 8.9% existed as a salt with acidic properties (calcium, magnesium, sodium, potassium cations).7 Although medications are frequently formulated as salts, the frequency and impact of patient confusion regarding salt form names is unknown.
Due to the high prevalence of pharmaceutical agents formulated as a salt and the potential for medication confusion by patients, the present study was conducted to evaluate patient knowledge and confusion related to medication salt forms for several common medications.
Methods
This study took place at Northeast Iowa Family Practice Center in Waterloo, IA, United States, a family medicine clinic staffed by 5 faculty physicians, 18 medical residents, 1 physician assistant, 1 clinical pharmacist, and 1 pharmacy resident. The clinic provides care to 7,912 patients and is the clinic home of the Northeast Iowa Family Medicine Residency Program, which is a community-based Family Medicine residency. Racial demographics of the clinic patient population include: 69% Caucasian, 15% African American, and 16% other minorities.
Patients were identified via prospective chart review prior to a scheduled appointment. Patients who were at least 18 years of age were approached by the primary investigator, schedule permitting, resulting in a convenience sample. Exclusion criteria included a diagnosis of dementia and/or a current prescription for medications approved to treat dementia. English fluency was not assessed. Patients were approached individually in the clinic waiting area between October 2011 and February 2012 and asked to complete an anonymous, 12-item paper-based questionnaire. The first four items collected categorical demographic information about the respondent’s age, gender, level of education, and number of current prescription medications. The next three questions specifically addressed patient knowledge of medication salt forms. Patients were asked to choose the active ingredient in “metoprolol succinate” by marking metoprolol or succinate. Uncertain also was an option. Next patients were asked if two medications named in a pair were the same by marking yes, no, or uncertain. The two sets of paired medications were benazepril and benazepril HCTZ and losartan and losartan potassium. The final five questions on the questionnaire asked the respondents’ level of agreement with a statement using a Likert scale. A scaled response was used for these items in order to allow variation in response strength. The first of these questions gauged the respondents’ level of confusion related to the names of their prescription medications. The next two questions assessed the level of agreement with the medical-related implications of two medications with an associated salt: glucosamine sulfate and warfarin sodium. These questions were: 1.) “If I had a “sulfa” allergy, I would be concerned about starting a medication named Glucosamine Sulfate”, and 2.) “If I were on a low-sodium diet, I would be concerned about starting a medication called Warfarin Sodium”. The final two questions in this section gauged the respondents’ assessment of the medication counseling received from their provider or pharmacist for new medications. The study was approved by the Wheaton Franciscan Healthcare Institutional Review Board, Milwaukee, Wisconsin.
Study analyses were performed using SPSS 20.0 statistics program (IBM Armonk, NY). Descriptive statistics were utilized to characterize the study sample and their responses to questions regarding medication salt forms. Multivariate linear regression was performed to identify the independent effects of demographics on the total number of correct responses given for 3 items about medication salt forms as previously described (metoprolol succinate, benazepril HCl vs. benazepril HCTZ, and losartan potassium vs. losartan). Only cases providing answers to all assessed items were included. p-values less than 0.05 were considered statistically significant.
Results
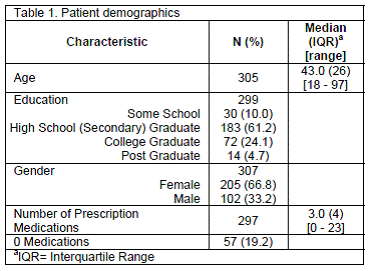
A total of 308 patients completed the questionnaire. The median age of our population was 43.0 (IQR=26) years (Table 1). Approximately two-thirds of respondents were female, 61.2% completed high school (secondary school), 28.8% completed college or postgraduate education, and the median number of prescription medications was 3.0 (IQR=4).
More than half of respondents (58.3%) were uncertain whether metoprolol or succinate was the active ingredient in the medication named “metoprolol succinate.” The remaining respondents were divided with 24.9% of respondents correctly answering metoprolol and 16.8% incorrectly answering succinate (Table 2). Approximately one-third of respondents (36.2%) were uncertain whether benazepril HCl and benazepril HCTZ were the same medication and a minority incorrectly reported the two medications were the same (6.9%). When the same question was asked of losartan and losartan potassium, 42.7% of respondents were uncertain while 12.0% correctly reported the medications were the same.
There was variation in the extent to which patients found their medication names to be confusing with 41% percent agreeing or strongly agreeing and 48% disagreeing or strongly disagreeing with the same statement (Table 3). Only 8.5% of respondents reported they should not be concerned about taking glucosamine sulfate if they had a “sulfa” allergy, which was the appropriate response. Uncertainty was common for these items. Similarly, only 16.3% reported they should not be concerned starting the medication warfarin sodium if they were told to be on a low sodium diet, which was the appropriate response. Most respondents agreed/strongly agreed that their prescriber and pharmacist discuss the name and use of new medications with them (87.8% and 90.1%, respectively).
Multivariate Results
Correct answers from items listed in Table 2 were summed to serve as the dependent variable for a multivariate linear regression with the range of 0 to 3 (Table 4). The number of cases included was 290 as 18 cases had at least one missing data item. Number of prescription medications and education level were positively associated (p<0.001, p<0.01, respectively) with correct responses to questionnaire prompts while age was negatively associated (p<0.001) with correct responses. Gender was not associated with more correct responses.
Discussion
The results from this questionnaire show that the majority of patients in the sample had limited understanding of the salt forms of common medications with most patients expressing uncertainty or incorrect reasoning related to interpreting salt forms of medications. Respondents struggled to identify the active ingredient from the salt form for metoprolol succinate and had uncertainty in their ability to differentiate benazepril HCL and benazepril HCTZ, and losartan from losartan potassium. There was also uncertainty over the implications of taking a salt form medication, both in regards to the dietary intake of that salt and in regards to medication allergies to similar sounding medications. This gap in health literacy has the potential to contribute to decreased medication adherence if patients inappropriately modify their regimens or discontinue doses to avoid problems perceived to relate to the salt form of their medications. This issue of salt form confusion adds a patient-level source of medication errors to the existing literature1-4 about prescribers and pharmacists making errors related to look-alike sound-alike medications.
Several demographic variables were identified as potentially relating to a patient’s ability to correctly interpret the salt form on a prescription label, although the strength of the finding was relatively small, especially given the restricted range of the dependent variable. Patients taking a greater number of medications were more likely to answer questions correctly compared to those on fewer medications. Taking more medications may have given these patients more experience with medications and their salt forms which they extrapolated to the cases on the questionnaires. Those with higher levels of education also were more likely to answer correctly. Increasing age was negatively associated with answering questions correctly, with older patients being less likely to answer correctly. These multivariate findings, however, should be interpreted as exploratory in nature and requiring further validation.
In the United States, the Institute for Safe Medication Practices (ISMP) recommends the salt not be included on prescription labels for generic medications unless there are multiple salt forms available.8 The pharmacy regulations of five U.S. States (Iowa, California, New York, New Mexico, and Virginia) were searched, but there was no mention of salt form rules or regulations.9-13 These five states were selected as a limited, convenience sample to determine if state pharmacy regulations address the inclusion/exclusion of medication salt forms on prescription labels. Inconsistencies resulting from a lack of regulation may result in patient confusion if they fill prescriptions at multiple pharmacies with differing policies on the inclusion of salt forms on medication labels. The FDA is now applying a policy adopted by USP for drug products that concerns the nonproprietary name and strength for drugs that contain a salt.14 This policy, “Monograph Naming Policy for Salt Drug Substances in Drug Products and Compounded Preparations” states that when an active ingredient in a drug product is a salt, the nonproprietary name of the drug product will contain the name of the active moiety (or neutral form) rather than the name of the salt.15 Some exceptions exist which allow the salt form to be retained in the name and strength.14,15
This results of our survey raise concern over how well medications are explained to patients. While the majority of patients agreed that their prescriber and pharmacist do an adequate job explaining their medications to them, the results suggest room for improvement and an apparent gap in medication education being provided by providers. If patients were counseled more extensively about the salt form in their medication name, they may have less uncertainty about the implications of taking that medication salt. However, this may create more errors, confusing patients who would have never paid attention to the salt form.
The most viable solution to this issue may be to standardize the use of salt forms on prescription labels. Removing the salt form, as recommended by ISMP, may be the most direct route to decreasing confusion. However, ISMP guidelines do recommend including the salt form when expressing a generic drug name if multiple salt forms are available.8 Such exceptions seem prudent for drugs when different salt forms confer different therapeutic properties (e.g. calcium acetate vs. calcium carbonate, hydroxyzine hydrochloride vs. hydroxyzine pamoate). If consistency could be established, the issue of patient confusion over salt forms could be better managed.
Limitations
The results of this study should be considered in light of several limitations. We assessed whether patients would be concerned in several circumstances, not whether they actually knew if there was a problem with taking the medication. Even then, a patient could be concerned about salt forms without rising to a level where the patient would actually alter their behavior, such as stopping a medication. Also, patients completing the questionnaire may have experience using the particular medication in question and thus may have had knowledge other respondents did not. Specific prior medication use, however, was not assessed or modeled. As noted, formal measures of English fluency or health literacy were not assessed among respondents. Our results may not be generalizable to other populations. This study was exploratory and used a convenience sample. The modest sample size may have been underpowered to detect significant differences in responses and limits the ability to generalize our findings to other populations.
Practice Implications and Future Research
Prescribers and other health care professionals should be made aware that salt form ambiguity could be an important source of confusion and relevant to the health literacy domain. The present findings support the need to further standardize the labeling of medication salt forms on prescription packaging and medication information for patients designed to decrease salt form confusion, particularly when the salt provides no useful information to the patient in differentiating the medication name. Further research to determine the frequency and impact of patient confusion regarding medication salt forms is warranted. The present questionnaire did not include a validated measure of health literacy, such as the Newest Vital Sign (NVS).16 Future research could test the relationship between the salt form knowledge questions and existing health literacy measures as a new health literacy indicator.
Conclusions
Medication salts can be a source of confusion for patients. While there are new measures by the FDA to improve salt form clarity on drug product labels from drug manufacturers, such measures may not be reflected on the labels of dispensed prescriptions from retail pharmacies. As health care providers, the option to explain a salt form on a prescription package during new prescription counseling should be considered. Standardization of the inclusion or exclusion of salt forms on prescription packages is an option that should be explored to decrease patient confusion about medication names.
Conflict of interest
All authors have signed the ICMJE Disclosure form and have no known or potential conflicts related to this work.
References
1. Hoffman JM, Proulx SM. Medication errors caused by confusion of drug names. Drug Safety 2003;26(7):445-452. [ Links ]
2. Kenagy JW, Stein GC. Naming, labeling, and packaging of pharmaceuticals. Am J Health-Syst Pharm 2001;58(21):2033-2041. [ Links ]
3. Filik R, Purdy K, Gale A, Gerrett D. Labeling of medicines and patient safety: evaluating methods of reducing drug name confusion. Human Factors 2006;48(1):39-47. [ Links ]
4. Gabriele S. The role of typography in differentiating look-alike/sound-alike drug names. Healthc Q. 2006;9(Spec No):88-95. [ Links ]
5. McDougall DJ, Lallier TS, Hoehns JD, Friedman RL. Medication nonadherence due to misunderstanding of the salt form. Ann Pharmacother. 2014;48(1):149-150. doi: 10.1177/1060028013510150. [ Links ]
6. Kumar L, Amin A, Bansal AK. Salt selection in drug development. PharmTech.com. 3/2/2008. http://pharmtech.findpharma.com/pharmtech/Peer-Reviewed+Research/Salt-Selection-in-Drug-Development/ArticleStandard/Article/detail/500407 (accessed May 31, 2015). [ Links ]
7. Paulekhun SG, Dressman JB, Saal C. Trends in active pharmaceutical ingredient salt selection based on analysis of the Orange Book database. J Med Chem. 2007;50(26):6665-6672 doi: 10.1021/jm701032y. [ Links ]
8. Principles of designing a medication label for community and mail order pharmacy prescription packages. https://www.ismp.org/tools/guidelines/labelFormats/comments/default.asp (accessed March 19, 2015). [ Links ]
9. Iowa Administrative Code. Iowa Board of Pharmacy. http://www.state.ia.us/ibpe/rules_laws/ (accessed March 30, 2015). [ Links ]
10. Laws and Regulations. California State Board of Pharmacy. http://www.pharmacy.ca.gov/laws_regs/ (accessed March 30, 2015). [ Links ]
11. Laws, Rules, and Regulations. NYSED.gov Office of the Professions. http://www.op.nysed.gov/prof/pharm/pharmlaw.htm (accessed March 30, 2015). [ Links ]
12. Pharmacy: Rules and Laws. New Mexico Regulation and Licensing Department. http://www.rld.state.nm.us/boards/Pharmacy_Rules_and_Laws.aspx (accessed March 30, 2015). [ Links ]
13. Virginia Board of Pharmacy Laws and Regulations. Virginia Board of Pharmacy. https://www.dhp.virginia.gov/pharmacy/pharmacy_laws_regs.htm (accessed March 30, 2015). [ Links ]
14. Kremzner ME, Lal R, Stodart B. FDA application of the USP salt policy. Am J Health-Syst Pharm 2014;71(9):694-696. doi: 10.2146/ajhp130507. [ Links ]
15. Unites States Pharmacopeia. Monograph naming policy for salt drug substances in drug products and compounded preparations. www.usp.org/usp-nf/development-process/compendial-nomenclature/monograph-naming-policy-salt-drug-substances-drug-products-and-compounded (accessed August 17, 2015). [ Links ]
16. Weiss BD, Mays MZ, Martz W, Castro KM, DeWalt DA, Pignone MP, Mockbee J, Hale FA. Quick assessment of literacy in primary care: the newest vital sign. Ann Fam Med 2005;3(6):514-522. doi: 10.1370/afm.405. [ Links ]
Received (first version): 08.10.15
Accepted: 03.02.16