INTRODUCTION
Vancomycin exhibits time-dependent antimicrobial killing. Ideally, the area under the curve/minimum inhibitory concentration (AUC/MIC) ratio is the best pharmacokinetic parameter to monitor vancomycin efficacy with a target AUC/MIC ratio of 400;1 however, obtaining a trough (pre-dose) serum concentration is more practical in clinical practice.2 A study has shown vancomycin serum concentrations of 8-9 mg/L in children correspond with an AUC/MIC ratio of 400.1
Some strains of MRSA have demonstrated increased MICs (from 0.5 to 1-2 mg/L). This progressive increase represents the “MIC creep” phenomenon, and has been hypothesized to result in sub-therapeutic serum levels for MRSA.3 For this reason, there has been renewed interest in vancomycin pharmacokinetics. Changing the paragraph as suggested will lead into the discussion of the IDSA guidelines nicely.
In 2009, the Infectious Diseases Society of America, the American Society of Health System Pharmacists, and the Society of Infectious Diseases Pharmacists published consensus guidelines for vancomycin dosing and patient monitoring during MRSA treatment for adults.1 Children were excluded from these recommendations, but more recent guidelines stated that levels of 15-20 mg/L could be considered in children with serious infections such as bacteremia, infective endocarditis, osteomyelitis, meningitis, pneumonia or severe skin and soft tissue infections due to MRSA.4 Additional sutides have shown levels of 8-9 mg/L are effective.1 Acceptable vancomycin serum drug levels in CHEO’s internal dosing guidelines include levels greater than 5 mg/L.
Nevertheless, vancomycin dosing and optimal target levels required in children with infections due to other methicillin resistant gram positive organisms (e.g. coagulase negative Staphylococci) or less severe infections remain controversial because of pharmacokinetic variability within pediatric populations. The lack of clarity on this issue is illustrated by the fact that there is still a wide variation in dosing regimens used and therapeutic levels targeted.5,6,7 Institution specific guidelines are presented in Table 1.
Table 1 Institution specific guidelines for vancomycin administration
| Age | Dose | Frequency |
|---|---|---|
| Neonates 0-14 days of life, <28 weeks gestational age | 20-22mg/kg | Every 24 hours |
| Neonates 0-14 days of life, 29-34 weeks gestational age | 20-22mg/kg | Every 18 hours |
| Neonates 0-14 days of life, >35 weeks gestational age | 20-22mg/kg | Every 12 hours |
| Neonates >14 days of life, <28 weeks corrected gestational age | 20-22mg/kg | Every 12 hours |
| Neonates >14 days of life, 29-34 weeks corrected gestational age | 20-22mg/kg | Every 12 hours |
| Neonates >14 days of life, >35 weeks corrected gestational age | 20-22mg/kg | Every 8-12 hours |
| Infants, age 1 month to <12 months of age | 15mg/kg | Every 6 hours |
| Children, age 1 and ≤ 12 years | 15mg/kg | Every 6 hours |
| Adolescents, age ≥ 13 years | 1000mg | Every 6-8 hours |
The purpose of this study was to evaluate the current initial vancomycin dosing regimens and the resulting trough serum concentrations achieved in the pediatric population at our institution. A secondary objective was to describe the prophylactic and therapeutic use of vancomycin in this cohort of patients.
METHODS
Study design and population
The study was performed exclusively at the Children’s Hospital of Eastern Ontario (CHEO), a 165-bed tertiary care pediatric hospital in Ottawa, Ontario, Canada. Data was reviewed retrospectively from inpatients aged 0 to 18 years who were given intravenous vancomycin between January 1 and December 31, 2013. Patients were identified through a drug utilization report obtained via the pharmacy computer system (G.E. Centricity®). Patients with acute or chronic kidney disease or injury, those on dialysis or patients who were experiencing ongoing acute kidney injury at the time of initiation of vancomycin therapy were excluded. The study was approved by the CHEO Research Ethics Board.
Review of medical record
Medical records were accessed to obtain the following data: patient demographics (age, sex, weight, and height), hospital unit, vancomycin indication and dosing regimen, length of therapy, laboratory and microbiology culture data, vancomycin serum concentrations (including their temporal relationship to the preceding or subsequent vancomycin dose, and number of doses administered prior to trough levels), and concomitant use of other nephrotoxic medications (aminoglycosides, diuretics, acyclovir, amphotericin B, non-steroidal anti-inflammatory drugs, angiotensin-converting enzyme inhibitors, methotrexate, and cyclosporine). Baseline and periodic serum creatinine (SCr) values were collected up to 1 week before the start of vancomycin therapy, when available, and up to 48 hours following the discontinuation of vancomycin therapy.
Patients were considered to have acute kidney injury during vancomycin therapy if they had an increase in serum creatinine of greater than 1.5 times their baseline value based on the KDIGO guidelines.8 Creatinine clearance was estimated using the Schwartz equation.9
Analysis
Patients included in this study were classified by age as follows: neonates (0-28 days postnatal age and premature children (<38 weeks gestational age) up to 10 weeks postnatal age), infants (1 month to <12 months of age), children (between 1 and ≤12 years), adolescents (≥13 years).10,11
Patients were categorized into those who received vancomycin for less than or equal to 5 days and greater than 5 days. Categorization of >5 days was chosen to allow for adequate time for empiric therapy to be discontinued as cultures are finalized within this time period. From the list of patients who received ≤5 days of therapy, a convenience sample of patients was chosen alphabetically, from a list of patient surnames. All patients who received vancomycin for greater than 5 days were evaluated and categorized according to treatment or prophylaxis.
Since 2010, the Children’s Hospital of Eastern Ontario has adopted a hospital wide, empiric initial vancomycin dosing recommendation: term neonates 20-22 mg/kg/dose every 8-12 hours, children >1 month 15 mg/kg/dose every 6 hours,. These guidelines are available on a hospital wide intranet, accessible to all prescribers. Acceptable trough vancomycin serum levels range from 5-20 mg/L and any level ≥5 mg/L is reported numerically.
Nephrotoxicity was assessed for all courses of vancomycin. For the purpose of the statistical analysis, use of one or more aforementioned nephrotoxic drugs was considered as a single categorical variable.
Data analysis was performed using SPSS, version 21. Patient demographics, vancomycin dosing, and resulting trough serum concentrations were analyzed using descriptive statistics. Continuous data were reported as median and interquartile range (SD), and categorical data were reported as numbers and percentages. An independent samples student t-test was used to compare means between 2 groups, while a Pearson’s chi squared test was performed to evaluate independence between categorical variables.
RESULTS
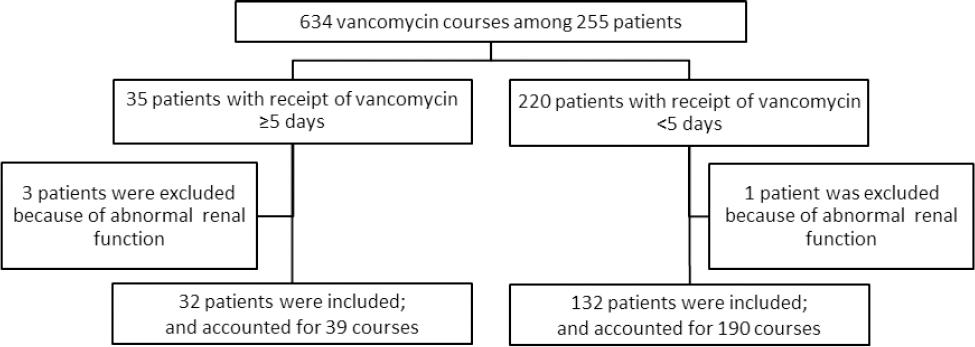
A total of 634 courses of intravenous vancomycin were prescribed to 255 children in 2013. Four patients with pre-existing chronic or acute kidney disease were excluded. Of the remaining 251 patients, 32 patients received vancomycin for >5 days. A convenience sample of patients who received courses of vancomycin of ≤5 days consisted of 132 patients. There was no difference with respect to age or gender between the patients in the convenience sample (n=132) and those excluded (n=87) (Figure 1).
Patients’ demographics included in the analysis are shown in Table 2. Of the 229 total courses, which accounted for 1111 days of therapy, the most common clinical indications stated for initial vancomycin use were febrile neutropenia 58 courses (25.2%), presumed sepsis/catheter-related infection 49 courses (21.3%), meningitis 33 courses (14.3%), skin or soft tissue infection 19 courses (9.2%), and pneumonia 20 courses (8.7%). Thirty-nine courses (21.3%) were given for other indications such as urinary tract infections, fever of unknown origin, or endocarditis. There were differences in indication between age groups. No infants or neonates received vancomycin for febrile neutropenia, while this indication accounted for 44.7% of stated indications in21 adolescents while sepsis/line infection was the indication stated for 56.1% of courses in 19 neonates. Overall, the mean duration of vancomycin therapy was 4.9 days (SD=19.4), with a median duration of 2.3 days and IQR of 2.11 in 164 patients. Of these, 153 (66.8 %) vancomycin courses were given for <3 days, 37 (16.2%) courses for 3-5 days, and 39 (17.0%) courses in 32 patients for >5 days. The mean duration of vancomycin is 1.86 (SD=1.16) in the convenience sample of 132 patients versus 9.73 days (SD=5.30) of patients on vancomycin for >5 days.
Table 2 Demographics and initial vancomycin dosing for all included patients.
| Neonates (n=32) | Infants (n=27) | Children (n=77) | Adolescents (n=28) | |
|---|---|---|---|---|
| Sex, % | ||||
| female | 43.8 | 44.4 | 42.9 | 46.4 |
| male | 56.3 | 55.6 | 57.1 | 53.6 |
| Location, % | ||||
| NICU | 75.0 | 7.4 | 0 | 0 |
| PICU | 3.1 | 29.6 | 18.2 | 25.0 |
| General Pediatrics | 21.9 | 63.0 | 58.4 | 50.0 |
| Oncology | 0 | 0 | 23.4 | 25.0 |
| Age | ||||
| median (range) | 16 d (1-66 d) | 124 d (29-329 d) | 5.1 y (1.0-12.9 y) | 15.6 y (13.0-18.5 y) |
| IQR | 24 d | 101 d | 5.8 | 2.1 |
| mean (SD) | 21 d (19 d) | 150 d (89 d) | 5.8 y (3.5 y) | 15.8 y (1.4 y) |
| Weight, kg | ||||
| median (range) | 2.7 (0.7-4.0) | 7.2 (3.1-12.3) | 17.5 (8.4-77.9) | 64.1 (32.0-109.4) |
| IQR | 1.3 | 3.1 | 12.5 | 22.82 |
| mean (SD) | 2.5 (0.9) | 6.9 (2.2) | 23.1 (14.1) | 66.0 (19.7) |
| Allergy to penicillin, % | 0.0 | 3.7 | 3.9 | 14.3 |
| Serum creatinine, µmol/L mean (SD) | 44.9 (18.8) | 27.9 (17.8) | 34.9 (13.2) | 64.7 (21.1) |
| Initial dosing, mg/kg/day | ||||
| median (range) | 40.8 (14.5-62.9) | 59.6 (39.5-62.8) | 59.7 (39.3-88.3) | 60.0 (25.0-70.2) |
| IQR | 12.8 | 2.9 | 1.7 | 12.32 |
| mean (SD) | 41.9 (12.6) | 57.7 (5.2) | 59.1 (4.7) | 55.6 (10.5) |
| Dosing interval, hours mean (SD) | 12.8 (5.0) | 6.2 (1.2) | 6.1 (0.4) | 6.2 (1.1) |
The neonatal population was defined as full-term newborns 0-28 days postnatal age and premature children (<38 weeks gestational age) up to 10 weeks postnatal age; patients aged 1 month to <12 months of age were classified as infants; patients between 1 and 12 years were classified as children; and patients between 13 and 18 years were classified as adolescents.
Overall, a total of 411 levels were drawn: in 43 (18.8%) courses, no level was drawn; in 84 (36.7%) only one level was drawn; in 56 (24.5%) two levels were drawn; in 20 (8.7%) three levels were drawn; and more than three levels were drawn in 26 courses (11.3%). The mean number of levels drawn in one course was 1.79 (SD 1.95). The number of levels drawn per course increased with the duration of therapy. Of the 411 levels collected, 372 (90.5%) were trough levels.
Of the 164 patients, 119 (72.6%) had vancomycin levels drawn. Eight (6.7%) were drawn before the second dose, and were excluded leaving 111 levels available for analysis. Of these, 56 (47.1%) were drawn before the third dose, 28 (23.5%) before the fourth dose, and 16 (13.4%) before the fifth dose and 11 (9.2%) were drawn before the sixth or greater dose. Overall, 19 (17.1%) neonates, 18 (16.2%) infants, 53 (47.7%) children, and 21 (18.9%) adolescents had initial trough levels that were analyzed.
The serum trough levels corresponding to the initial doses are shown in Figure 2. In neonates (n=19), 40 mg/kg/day and 60 mg/kg/day dosing regimens were both used, respectively in 12 (63.2%) and 6 (31.6%) patients, while one (5.3%) received a dosing of less than 30 mg/kg/day. Only 2 patients (10.5%) did not achieve trough levels ≥5mg/L, they received doses of 20 mg/kg/day and 40 mg/kg/day.
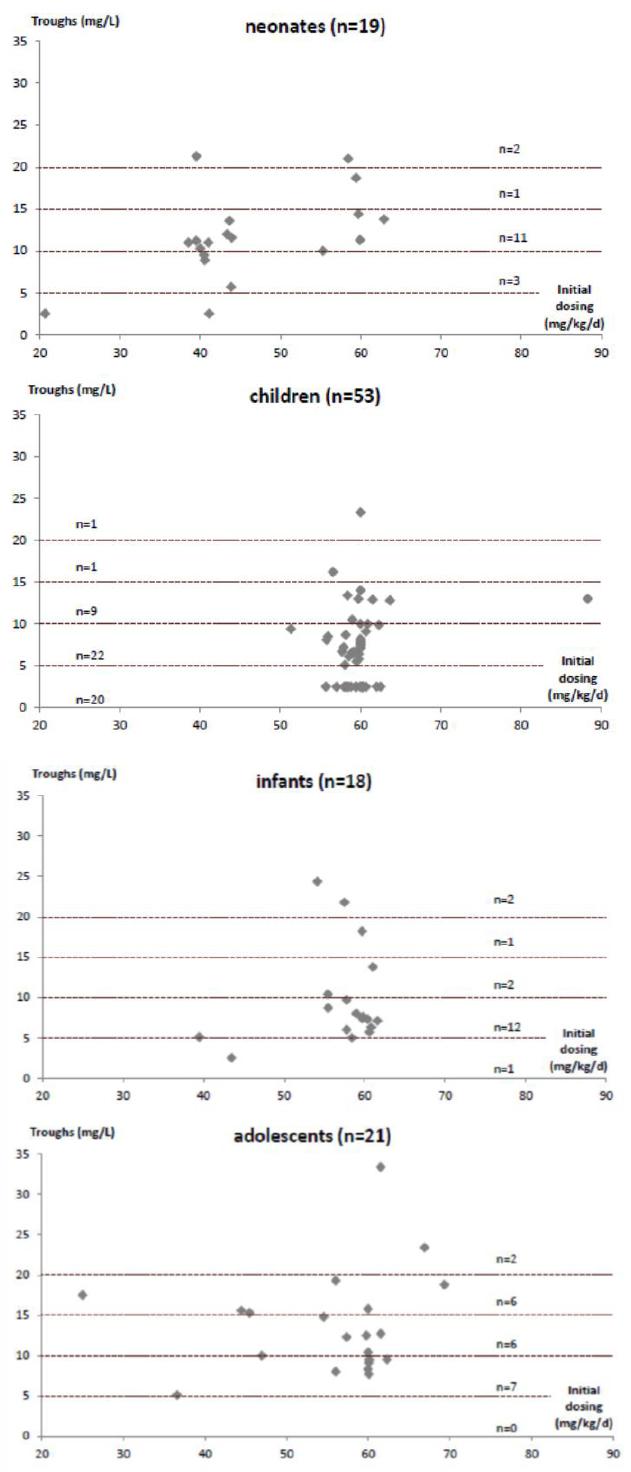
Figure 2 Distribution of initial vancomycin serum through concentrations achieved regarding initial dosing
For infants (n=18), 16 (88.9%) received an initial dose of 60 mg/kg/day. These 16 patients achieved a mean vancomycin level of 10.46 mg/L. The 2 patients (11.1%) who had trough levels of <5 mg/L both received 40 mg/kg/day of vancomycin as initial dosing.
For children (n=53), the dosing recommendation of 60 mg/kg/day was used in 52 patients (98.1%). Of these, 20 patients (37.7%) had initial vancomycin trough serum levels <5 mg/L. One oncology patient who had already received vancomycin prior to the study period was administered an initial dose of 90 mg/kg/day. Further analysis of this group revealed that both patient weight and age were significantly lower (p=0.005, p=0.006, respectively) in the group of children with troughs <5 mg/L (17.8; SD=7.7 kg and 4.5; SD=2.9 years) compared to the group of children who achieved through levels of ≥5 mg/L (28.3; SD= 17.6 kg and 7.2; SD=3.5 years). There was no statistically significant difference (p=0.152) in eGFR (available for 25/53 patients) between the group of children with troughs <5 mg/L (202; SD=67 mL/min/1.73 m2) and the group of children who achieved through levels of ≥5 mg/L (169; SD=42 mL/min/1.73 m2). In the children group, oncology patients were not more likely to achieve trough levels <5 mg/L compared to non-oncologic patients (chi-square test, p=0.831). In the adolescent age group (n=17), the oncology patients were also not more likely to achieve trough levels <5 mg/L compared to non-oncology patients (independent sample t test p=0.884). Also, there was no association between levels drawn before the third dose and levels <5mg/L (chi-square test, p=0.663).
Among adolescents (n=21), all patients had trough levels of >5 mg/L using various dosing regimens. Eleven (52%) received 60 mg/kg per day, 5 (24%) received 50 mg/kg per day, 3 (14%) received 40 mg/kg per day and 2 (10%) received less than 40 mg/kg per day (35 mg/kg per day and 18 mg/kg per day). The five patients that received 50 mg/kg per day weighed more than 80 kg and were administered the maximum dose of 1g q6h for adults.
A total of 39 courses of vancomycin in 32 patients lasted for greater than 5 days. Of those, 24 (61.5%) were administered in patients with documented infections and 15 (38.5%) with no specific bacterial pathogen isolated (Table 3).
Table 3 Clinical characteristics of patients who received >5 days of vancomycin.
| Bacteria isolated and type of infection | N | Therapy duration, days | ||
|---|---|---|---|---|
| Mean (SD) | Median | Range | ||
| MRSA* | ||||
| Bacteremia | 2 | 21.9 (8.8) | 21.9 | 13.1 – 30.8 |
| SSTIs ~ | 1 | 7.0 (-) | - | - |
| Coagulase negative Staphylococcal species | ||||
| Line infection | 12 | 12.1 (6.0) | 10.3 | 6.5 – 28.2 |
| Foreign body infection | 3 | 83.3 (118.8) | 17.5 | 9.4 – 289.0 |
| alpha-hemolytic Streptococcus, penicillin-I/R ƙ | ||||
| Bacteremia | 3 | 13.1 (5.9) | 11.5 | 6.7 – 21.0 |
| Urinary tract infection | 1 | 13.0 (-) | - | - |
| Enterococcus ampicillin-S | ||||
| Bacteremia (penicillin allergy) | 1 | 15.8 (-) | - | - |
| Rothia mucilaginosa | ||||
| Line infection | 1 | 18.3 (-) | - | - |
| Total | 24 | 25.5 (55.4) | 12.3 | 6.5 – 289.0 |
| No bacteria isolated | ||||
| Febrile neutropenia therapy | 4 | 8.0 (2.2) | 6.9 | 6.5 – 11.8 |
| Treatment for presumed infection | 3 | 8.3 (3.5) | 6.0 | 5.8 – 13.3 |
| Presumed neonatal infection ¥ | 8 | 7.1 (1.0) | 6.8 | 5.7 – 8.6 |
| Total | 15 | |||
*Methicillin resistant Staphylococcus aureus.
~SSTIs = skin or soft-tissue infections.
ƙPenicillin-I/R = intermediate susceptibility or resistant to penicillin.
¥Presumed sepsis/line infection, necrotizing enterocolitis, or meningitis.
The 24 cases in which vancomycin was used to treat gram positive infections accounted for an absolute number of 532 days of vancomycin therapy (47.9% of the total 1111 days of vancomycin therapy analyzed in this study). All patients who had treatment for culture confirmed infections had negative cultures following initiation of treatment. Four patients were found to have methicillin-resistant Staphylococcus aureus infections (MRSA), all isolates had MICs less than 1 mg/L. One patient, who was treated successfully for MRSA bacteremia, confirmed by negative blood cultures, had levels greater than 5 mg/L but died after 13 days of vancomycin therapy of an unrelated cause.
Among the 229 courses of vancomycin given, one or more nephrotoxic drugs were administered concomitantly in 182 (79.5%) courses. The most common co-administered agents were nonsteroidal anti-inflammatory drugs 52 (28.6%), antivirals 47 (25.8%), gentamicin 46 (25.3%) and furosemide 22 (12.1%). Other agents included amphotericin B in 17 (9.3%), angiotensin-converting enzyme inhibitors in 1 (0.5%) and methotrexate in 2 (1.1%).
Thirteen of the 229 vancomycin courses (5.7%) may have led to nephrotoxicity, with a median serum creatinine increase of 84% (max 376%) of baseline value. Six of the 13 patients who experienced nephrotoxicity had a least one trough level >20 mg/L during therapy. In 11 courses, nephrotoxic drugs were administered concomitantly (Table 4).
Table 4 Concomitant nephrotoxic agents administered to patients who experienced nephrotoxicity
| Case 1: acyclovir |
| Case 2: acyclovir, gentamicin, furosemide |
| Case 3: furosemide, pip/tazo |
| Case 4: pip/tazo |
| Case 5: amphotericin B |
| Case 6: furosemide |
| Case 7: acyclovir |
| Case 8: furosemide |
| Case 9: gentamicin, amphotericin B, cloxacillin |
| Case 10: methotrexate, cytarabine, pip/tazo |
| Case 11: gentamicin, amphotericin B, pip/tazo |
Of the 32 patients who received greater than 5 days of therapy, 5 (15.6%) experienced a serum creatinine increase during the vancomycin therapy (greater than 1.5 times the baseline level). Among these patients, 4 received concomitant nephrotoxic drugs and 3 had at least one trough level higher than 20 mg/L. The mean percentage of serum creatinine increase was 136.6 (SD 119.8) and the increase ranged from 50% to 344%.
DISCUSSION
In this study, we reviewed the initial vancomycin dosing regimens and resulting vancomycin trough concentrations in 164 pediatric patients with normal renal function.
The median initial dosing for vancomycin in children greater than one month of age was approximately 60 mg/kg/day which is consistent with the current recommendation for empiric vancomycin dosing in patients older than one month.4 Nearly 81% of our patients received an initial vancomycin dosing in a range from 50 to 70 mg/kg per day. Of the patients who received initial vancomycin dosing of 50-70 mg/kg per day, 77.8% had a trough level greater than 5 mg/L meeting our institution’s goal of 5-20 mg/L.
Of the groups studied, only the children between 1 and 12 years did not consistently have levels >5 mg/L. In this group, about one third did not achieve detectable levels. Patients with lower weight (p=0.005) and lower age (p=0.006) were statistically more likely to have sub therapeutic levels. The most likely pharmacokinetic parameters causing low levels are the volume of distribution and clearance. The volume of distribution may be increased due to larger extracellular fluid stores in younger children. Disease states such as oncological disorders or cystic fibrosis may increase the volume of distribution. However in this study, oncologic patients were not more likely to achieve a trough level <5 mg/L than non-oncologic patients (p=0.831). Children have shorter half-lives (2-3 hours) than adults (5-11 hours) resulting in increased clearance of vancomycin and lower serum trough levels.
Previous studies have identified this age group as having difficulty obtaining adequate levels. Benner et al reviewed multiple dosing regimens for pediatric patients aged 1-18 years. Fifteen mg/kg per dose given every 6 hours more consistently resulted in targeted trough concentrations of 5-15 mg/L. The study population included all pediatric patients; however, it did not take into account pharmacokinetic changes of the developing child.12 Another study by Broome and colleagues reviewed vancomycin dosing in all pediatric patients under the age of 18 years but also grouped patients according to their population pharmacokinetic half-lives for vancomycin. Even though this study used vancomycin dosing regimens of 15 mg/kg per dose every 8 hours, they found significant trough variations in the children’s group, 2-12 years of age versus other groups: infants, 1-23 months, and adolescents, 13-18 years.5 This study is most closely aligned with our study; further research is warranted to review children between the ages of 1and 12 years.
In neonates, 40 mg/kg/day and 60 mg/kg/day dosing regimens were both used. Dosing in neonates should be considered separately; changes in body weight and/or gestational or postnatal age may explain the variation, which can potentially lead to an increased half-life of the drug. Due to this large inter-patient variability, it is difficult to provide standardized recommendations for this population. The current guidelines do not make recommendations for this population, therefore, dosing guidelines differ extensively depending on the source and institution.5,13 In infants, the initial dosing prescribed was globally in accordance with the recommendation of 60 mg/kg per day resulting in levels >5 mg/L.
We observed a low frequency of MRSA infections, all of which had MICs of less than 1mg/L. The number of patients in our study is too small to draw conclusions but Le et al and Frymoyer et al. used pharmacokinetic modeling to show that levels ≥5 mcg/mL equivalent to 5 mg/L are sufficient if MICs are 1 mg/L or less.1,14
In our study, over half of the total number of days of vancomycin was used for empiric therapy. In this group, the overall incidence of nephrotoxicity was 5.7%. Concomitant nephrotoxic agents, such as gentamicin, furosemide, acyclovir or amphotericin B, were often administered concurrently. Additionally, 6 of 13 patients who experienced nephrotoxicity had at least one supra-therapeutic vancomycin level >20 mg/L. Very few studies have evaluated the toxicity of vancomycin with other nephrotoxic drugs other than aminoglycosides. A majority of nephrotoxicity that was associated with vancomycin plus concomitant nephrotoxic agents occurred on the first day of vancomycin therapy. Most studies report vancomycin-associated nephrotoxicity generally occurring after several days of receipt of drug and the relationship between vancomycin serum concentrations, the presence of other nephrotoxic drugs and incidence of nephrotoxicity remains unclear but suggest that other drugs may be important contributors.15,16,17 This is important to note for harm reduction since slightly over a third of the longer courses were given to patients who had no documented Gram-positive bacterial infections.
Our study is limited in that we looked only at the initial trough levels to assess the empiric dosing of vancomycin, but dose adjustments were not assessed. Secondly, we did not evaluate the vancomycin trough concentration variation if it was drawn prior to the third, fourth, or fifth dose. This would have enabled us to determine time to steady state. Rates of MRSA are low at our institution, enabling us to continue to target levels of 5 mg/L for most infections however this may not encompass all pediatric institutions. In addition, the convenience sample may not have been representative of the entire cohort.
CONCLUSIONS
In conclusion, the findings of this study suggest that in our institution, with a low rate of MRSA, initial empiric vancomycin dosing of 60 mg/kg per day or adult dosing 1 g every 6 hours was associated with achievement of levels that are greater than 5 mg/L. Further prospective studies are required to better predict levels using empiric dosing regimens especially in children aged 1-12 years of age.