INTRODUCTION
Warfarin remains a major choice for oral anticoagulation in non-valvular atrial fibrillation and deep vein thrombosis; however, anticoagulation status is impacted by many factors including dietary, ethnicity, genetics, age and weight.1,2,3,4To ensure adequate efficacy and safety with warfarin therapy, regular assessment is required to maintain an International Normalized Ratio (INR) within the recommended therapeutic range of 2.0 - 3.0, except for patients with mechanical heart valves or recurrent myocardial infarction, where the recommended range is 2.5-3.5.5
Optimising the percentage of time spent in therapeutic range for INR (TTR) can reduce the risk of haemorrhagic and thromboembolic events.6,7Connollyet al.8reviewed TTR data from anticoagulation centres of 15 countries and reported that a TTR of at least 65% is required for optimum benefit of warfarin therapy. This threshold forms part of the guidelines supported by the United Kingdom National Institute for Health and Care Excellence and the University of Nottingham PRIMIS development group9; furthermore these guidelines suggest that there is no benefit from warfarin therapy when TTR <40%.10,11
Active monitoring through specialized anticoagulation clinics have proven beneficial in optimizing warfarin therapy and reducing the risk of complications.12,13,14,15,16In a systematic review published in 2017 of 25 randomized controlled and observational studies of 12,252 patients receiving warfarin therapy, it was shown that anticoagulation status was significantly improved in pharmacist managed outpatient care facilities, including lower bleeding and thromboembolic events.17
At the first pharmacist managed anticoagulation clinic established in 2013 at the Cornwall Regional Hospital in Jamaica, patients are managed for INR control once warfarin therapy is initiated by physicians at the hospital. The role of the pharmacist assigned to the clinic include making dosage adjustments to warfarin therapy as needed, as well as teach patients on appropriate dietary practices with specific focus on vitamin K content. Possible drug interactions are also reviewed and changes actioned after consultation with assigned physician. The pharmacist initially measures INR weekly until target INR is attained and then appointments are moved to once monthly. The aim of this study was to assess the anticoagulation status achieved by this pharmacist managed clinic over a six month period of monthly monitoring.
METHODS
A retrospective review of patient records of the pharmacist managed warfarin clinic at the Cornwall Regional Hospital from the period January 2014 to December 2016 was done. The Cornwall Regional Hospital is a 400-bed capacity institution, providing many specialty services including Cardiology. The protocol was approved by the Ministry of Health Ethics Committee and the University of the West Indies Ethics Committee.
Only records of patients who were of at least 18 years old at first registration to the clinic and were on warfarin therapy for at least six months were selected. Six months was chosen as adequate to assess clinic performance based on a previous study evaluating anticoagulation clinics of the Veteran Affairs Health System in the United States of America.18Records were excluded if patients were younger than 18 years, had severely impaired liver and kidney function, as well as patients with bleeding disorders such as hemophilia. Data extracted from the records included patient age, gender, indication for warfarin, warfarin dose at first visit and at each monthly visit, as well as the number of missed warfarin doses per week. The missed dose information was used as a measure of compliance with warfarin therapy. INR readings were also recorded from the patient records. These readings were obtained by the pharmacist at the each visit using the Roche Diagnostic CoaguChek XS Plus system; a validated point-of-care device that consists of a CoaguChek XS Plus monitor with CoaguChek XS Plus pro-thrombin test strips and produces results within seconds from a drop (≥ 8microL) of capillary blood. The test strip consist of lyophilized reagents, thromboplastin and a peptide substrate and has an INR measuring range from 0.8 to 8.0 with 97% accuracy when compared to lab results.19,20INR extracted from the records included the INR reading at the first visit and INR reading by month for six months.
Data Analysis
Continuous variables were characterized by mean and standard deviation [SD] and by median and interquartile range [IQR]; categorical variables were characterized by frequencies and percentages. TTR was calculated using the Rosendaal linear interpolation method, which assumes there is a linear relationship between two consecutive INR results.21This method determines the proportion of time for which the INR is within therapeutic range of 2.0 - 3.0. TTR was calculated by month for a period of six months with month defined as a scheduled visit at 28 to 30 days of last clinic visit. Wilcoxon Signed Rank test was used to compare median percentage TTR between months.
Patients returning to the clinic after 30 days were considered as missing scheduled visit. Spearman’s rho co-efficient was used to analyse association between number of missing visits and TTR.
Further analysis involved comparing the change in anticoagulation status of patients between month three and month six. These periods were used to compensate for missed patient visits. Where patients missed the three month or six month appointment the last TTR value was carried forward to determine anticoagulation status categorization. Patients missing two consecutive month visits were excluded from the analysis of anticoagulation status.
Using guidelines established by the University of Nottingham PRIMIS development group, patients were stratified into categories based on TTR as poor anticoagulation status (TTR<40%), moderate anticoagulation status (TTR of 40 to 64%) and good anticoagulation status (TTR≥65%). McNemar-Bowker pairwise Chi-Square test for proportions was used to compare anticoagulation status at month three and month six. For all inferential statistics done, statistical significance was considered as p values less than 0.05.
RESULTS
In total, the study identified 52 patient dockets meeting inclusion criteria and the demographics are presented inTable 1. Thirty-six (69.2 %) patients were males and the ages ranged from 23 to 90 years with a median age of 58 years. At the first visit to the clinic, the warfarin weekly doses ranged from a low of 15.0 mg to 85 mg; 9 (17.3%) patients presented with an INR within the target range; 24 (46.2%) patients were below target INR and 19 (36.5 %) patients were above target INR. The most common indication for warfarin therapy was deep vein thrombosis. During the first month the weekly dose of warfarin prescribed by the clinic range from a low of 17.5 mg to a high of 130.0 mg and varied over the five to six month period from a weekly low dose of 15.0 mg to a high of 110.0 mg. Over the six months of data collected, the records documented 30 patients as being fully compliant with dosing, while 12 patients dockets noted one to three times missing warfarin dose for the month; information documenting patient compliance was missing for 10 patients.
Table 1. Details of the 52 patients in study population
| Num. patients | Mean (SD) | Median [IQR] | |
|---|---|---|---|
| Gender | |||
| Male | 36 | ||
| Female | 15 | ||
| Missing data | 1 | ||
| Indication | |||
| Deep vein thrombosis (DVT) | 22 | ||
| Atrial fibrillation | 18 | ||
| Pulmonary embolism (PE) | 5 | ||
| DVT and PE | 3 | ||
| DVT and Antiphospholipid syndrome | 1 | ||
| Chronic venous insufficiency | 1 | ||
| External rectal vein thrombosis | 1 | ||
| Cardiovascular accident | 1 | ||
| Age | 52 | 59 (16.4) | 58 [72-47] |
| Warfarin Weekly dose/mg (range: lowest-highest) | |||
| At first visit (Range: 15.0-85.0) | 38.1 (15.4) | 37.5[42.5-30.0] | |
| Month 1[Range:17.5-130.0] | 55.0 (20.4) | 52.5 [63.0-40.6] | |
| Months 2-6 [Range:15.0-110.0] | 46.8 (17.4) | 44.0[57.5-35.5] | |
| INR at first visit ( Target Range 2-3 ) | |||
| Below Target | 24 | 1.46 (0.28) | 1.47 (0.51) |
| Within Target | 9 | 2.32 (0.25) | 2.29 (0.27) |
| Above Target | 19 | 4.48 (1.54) | 3.59 (1.90) |
| TTR% | |||
| Month 1 | 51 | 36.5 (30.1) | 31 [52-10] |
| Month 2 | 51 | 54.2 (35.3) | 60 [82-23] |
| Month 3 | 49 | 53.3 (35.0) | 50 [93-21.5] |
| Month 4 | 47 | 53.4 (37.0) | 51 [100-18] |
| Month 5 | 48 | 53.4 (39.7) | 51.5 [100-15.5] |
| Month 6 | 40 | 56.4 (39.6) | 52.5 [100-20.5] |
| Total number of missed visits =26 |
SD=Standard Deviation; IQR=Interquartile Range; INR=International Normalized Ratio % TTR=Percentage time spent in therapeutic range
Of the total, 51 patients returned to the clinic within one month of the first visit with 40 (76.9%) patients attending the clinic two or more times in the first month. In terms of anticoagulation status, after one month, of the patients that were below target INR range at the first visit, 2/24 returned in good anticoagulation status, of those in target INR range, 4/9 returned in good anticoagulation status and of those above target INR range, 2/18 returned in good anticoagulation status. The mean and median TTR after one month were 36.5% (SD 30.1) and 31% [IQR 62-10], respectively (Table 1). Significant improvement in TTR was attained by the second month (54.2; SD 35.3 and 60 [IQR 82-23] respectively; p=0.001).
A total of 26 missed scheduled visits (of 312 visits; 8.3%) were recorded ranging from one patient in months one and two, three patients missing visits in month three, five patients in month four, 4 patients in month five and 12 patients in month six. No correlation was found between missed scheduled visits and median TTR by month. One patient missed two consecutive visits and was excluded from the assessment of anticoagulation status.
The change in anticoagulation status from month three to month six for the 51 remaining patients is displayed inFigure 1. At month three, 19 (37.3%) patients had good, 15 (29.4%) had moderate and 17 (33.3%) had poor anticoagulation status. Of those in good anticoagulation status at month three, 8/19 continued to remain in good anticoagulation status, while 7/19 presented with moderate and 4/19 deteriorated to poor anticoagulation status at month six. For patients moderate at month three, 6/15 improved to good anticoagulation status, 2/15 remained the same and 7/15 deteriorated to poor at month six. Of the seventeen patients that were in poor anticoagulation status at month three; there was improvement in TTR at month six for a greater proportion of the patients with 9/17 improved to good and 3/17 to moderate status; 5/17 remained in poor anticoagulation status. There was overall no significant improvement in the anticoagulated status of the patients from month 3 to month 6 (chi-square=3.60; degrees of freedom=3, p=0.31).
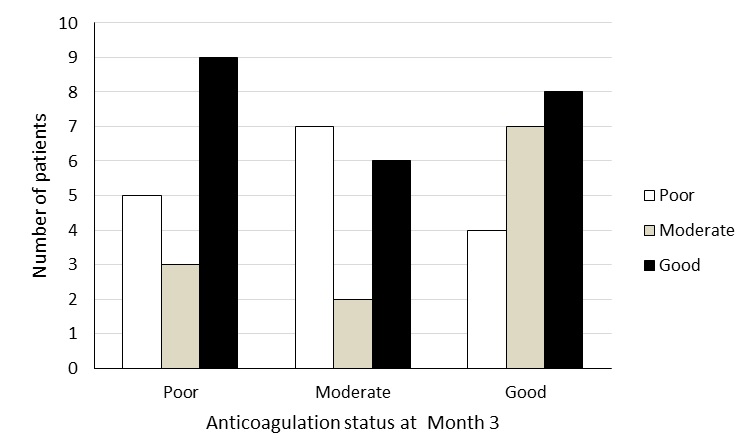
Figure 1 Change in anticoagulation status of patients on warfarin therapy from month three to month six; bars represent anticoagulation status at month six.At month three the proportions of patients in good, moderate and poor anticoagulation status were 19, 15, 17 respectively. By six months the proportions changed to 23, 12 and 16 respectively. With some patients improving and some deteriorating, the overall anticoagulation status remained the same from month three to month six (McNemar-Bowker pairwise chi-square=3.60; degrees of freedom=3; p=0.31).
DISCUSSION
A six month assessment of the first established pharmacist managed anticoagulant clinic in Jamaica showed that overall the clinic was successful in achieving good compliance with scheduled visits. In this study, the mean TTR obtained after one month was below values established as clinically adequate; but comparable with findings from other studies showing more time requirement.14By the second month of therapy, the clinic was successful in maintaining monthly mean TTR in the range 53.4 % to 56.4%. The level of anticoagulation control obtained in this current setting is comparable with the meta-analysis study of Erkens, Cate, Büller and Prins, 2012, which reviewed forty randomized controlled and cohort studies of 26,064 similar patients published between January 1990 and May 2012.22The mean TTR calculated from this meta-analysis ranged from 56% to 75%, with the higher percentage obtained when the first month was excluded from the calculations. Similar findings were reported in the systematic review by Manzooret al.17
Furthermore, the study of Erkenset al., proved that mean TTRs greater than 75 % were only observed after more than six months on warfarin. Therefore, while improvement in TTR was observed from month one to month three in the patients of this study, the absence of further improvement in patient anticoagulation status from month three to month six is consistent with the findings of Erkenset al.
The collection of the data for this study was limited by patients not attending scheduled visits, resulting in TTR values having to be carried forward for some patients. The measure of compliance with therapy was another limitation, as in some cases the information on missed dose was not available; furthermore patient recall over a month can be unreliable.
CONCLUSIONS
In conclusion, the findings suggest that the monthly monitoring in this pharmacist managed clinic attained moderate to good anticoagulation status for most patients within three months and sustained for up to six months. The anticoagulation control is comparable to similar out-patient settings and such services have reported benefits to efficacy, safety and cost.17,23,24Therefore, this pharmacist managed warfarin clinic services has the potential to facilitate better patient outcomes and should be supported.














