INTRODUCTION
Vancomycin is a glycopeptide antibiotic that has been in clinical use since 1958.1 Despite its frequent use, gaps still exist in our knowledge of optimizing therapy and avoiding adverse events in patient care. The area-under the curve to minimum-inhibitory concentration (AUC/MIC) dosing method has been identified as the most appropriate monitoring target for vancomycin.1 Previously, the AUC/MIC ratio has been cumbersome to calculate, and monitoring with targeted trough levels of 15-20 mg/dL as a surrogate marker for AUC was recommended.1 However, additional research has shown that trough-based monitoring is not a sufficient surrogate marker for AUC/MIC targets.2 3-4 Target AUC/MIC levels can be achieved without a trough concentration of 15-20 mg/dL.2 Therefore, current expert consensus recommends an AUC/MICBroth-Micro-Dilution target of 400 to 600 to achieve clinical efficacy while improving patient safety.1
Vancomycin-associated acute kidney injury (AKI) occurs in 5-43% of treated patients.5 AKI has been shown to significantly decrease long-term survival rates, increase morbidity and prolong hospitalizations in critically ill patients.6 Literature suggests risk of AKI increases with increasing vancomycin exposures and trough concentrations (>15-20 mg/dL), and there is additional evidence that AKI risk increases when daily AUC exceeds 700-1300 mg*hr/L.5,7-8
A 2017 retrospective study by Zasowski analyzed 323 patients receiving vancomycin for bacteremia or pneumonia for at least 72 hours.9 After excluding patients’ confounding risks for decline in renal function, such as Elixhauser comorbidity index and receipt of IV contrast dye, rates of nephrotoxicity were significantly higher in patients who received a concomitant nephrotoxin, and patients with AUC≥677 mg*hr/L.9
Two approaches exist for monitoring AUC/MIC, the use of Bayesian software programs to estimate the 24-hour area under the curve (AUC24) with minimal pharmacokinetic sampling, or the use of two concentrations (peak and trough) and simple PK equations to estimate AUC24 values.1 The Bayesian approach provides some advantages. It provides accurate estimates of AUC24 values with trough-only sampling, however, given limited data it is recommended to be used with two vancomycin concentrations.1,10 A major disadvantage to this approach is the costly nature of the software programs. The advantage of using two concentrations is it is simpler and relies on fewer assumptions than the Bayesian approach. The main limitation of this approach is it is not adaptive like the Bayesian approach, and works best when levels are obtained at/near steady state.4,11
The purpose of this study was to compare mean initial 24-hour vancomycin exposure using traditional trough-based dosing versus dosing recommended by an electronic AUC/MIC dosing program.
METHODS
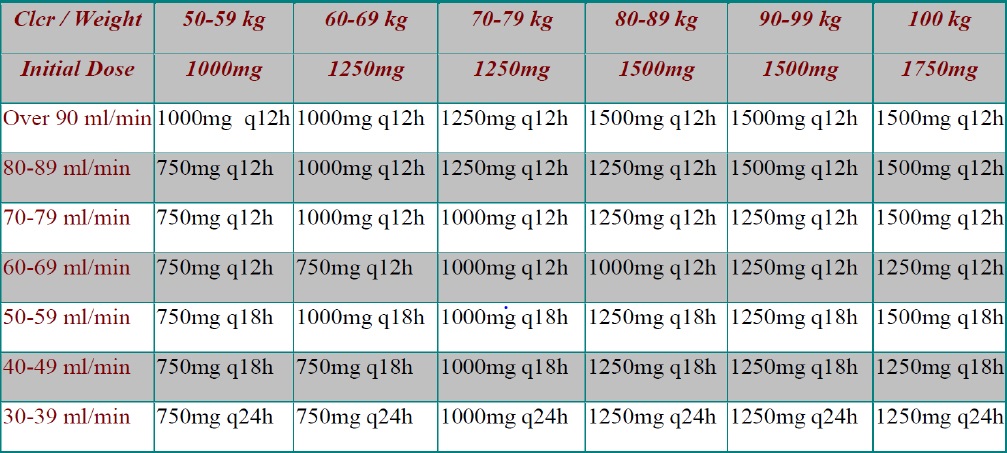
The single-center, retrospective cohort study was conducted at Cape Fear Valley Medical Center in Fayetteville, North Carolina, a 670-bed community hospital. Vancomycin dosing and monitoring is accomplished via pharmacy to dose consult service. Initial dosing regimens and therapeutic adjustments are determined per a hospital-wide nomogram (Figure 1) or utilizing first-order kinetic equations with goal trough concentrations of 10-20 mg/dL. Trough concentration goals are specific to infection location with lower trough targets of 10-15 mg/dL utilized for less severe infections such as skin and soft tissue infections and urinary tract infections. Trough concentrations of 15-20 mg/dL are used for all other infections. Therapeutic monitoring is based on trough-only serum levels. The institutional review board granted exempt status for this study.
Patients were identified via a report run in Epic© (electronic medical record) of inpatient vancomycin orders for patients admitted to a general medicine service from May 1, 2019 to December 31, 2019. To be included in the analysis, patients needed to be at least 18 years of age and have been treated for a suspected or documented infection with vancomycin for at least 24 hours. Patients were excluded if they were admitted to the intensive care unit during hospitalization, required hemodialysis or had unstable renal function on admission (increase of at least 0.3 mg/dL or 50% in SCr from known baseline), were treated for meningitis, were pregnant or received only one dose of vancomycin in the emergency department or for an indication of surgical prophylaxis.
The primary outcome was to compare mean total first day vancomycin dose between recommended AUC/MIC dosing and traditional trough-based dosing. For this endpoint each patient served as their own control by analyzing both the actual dose received and the dose recommended by the electronic AUC/MIC program. Secondary outcomes included comparing mean predicted AUC/MIC and trough concentrations between patients who received doses consistent with AUC/MIC recommendations versus those who did not. For these secondary endpoints, patients were assigned to groups based on whether they received dosing which was consistent with AUC/MIC recommended dosing or did not (trough-based dosing). Doses were considered consistent with AUC/MIC recommended dosing if the calculated AUC/MIC associated with the dose was between 400-600. We also sought to describe rates of vancomycin induced adverse events such as vancomycin induced acute kidney injury (defined as an increase in SCr level of at least 0.5 mg/dL or a 50 % increase from baseline in consecutive daily readings or a decrease in calculated CrCl of 50 % from baseline on two consecutive days in the absence of alternative explanation), Red Man syndrome and allergic reaction. A validated online calculator was used to calculate recommended AUC/MIC dosing, predicted AUC/MIC and trough values. This calculator utilizes published pharmacokinetic equations and principles to estimate a vancomycin dosing regimen for a patient utilizing body weight and creatinine clearance. After a regimen is calculated each step utilized in the calculations is available for the clinician to review for accuracy.12 Trough-based dosing was calculated using Cape Fear Valley’s nomogram, which recommends dosing based on creatinine clearance and weight in kilograms, (Figure 1) or utilizing first-order kinetic equations, based on pharmacists’ clinical judgement, if the patient did not meet parameters for the nomogram. Data collected included: patient demographics (gender, age, height, weight, body mass index, SCr and estimated creatinine clearance), vancomycin dosing information (indication for therapy, dose, frequency, total initial 24-hour vancomycin received and serum concentrations), and concomitant nephrotoxin use (piperacillin/tazobactam, loop diuretics, IV contrast dye, and ACEi/ARB). Vancomycin MICs were assumed to be <1 mg/L for this study as culture data was not collected. Data was collected by two investigators and checked by another investigator for completion and accuracy following the initial 10 entries as specified in the protocol. Data were input into Microsoft Access (Redmond, WA) and stored on secured network computers.. Categorical variables were compared using Pearson’s chi-square test. Continuous variables were compared using the Student t-test. A p-value of less than 0.05 was considered statistically significant. All statistical tests were run using JMP-14 Pro (SAS. Cary, NC).
RESULTS
Of the 619 patients evaluated, 264 (42.6%) met inclusion criteria. Patients were excluded for the following: surgical prophylaxis (n=45), treatment for less than 24 hours (n=273), unstable renal function (n=5), hemodialysis (n=21), treatment for meningitis (n=8), and pregnancy (n=1). Two patients were also excluded due to having documented allergies to vancomycin, vancomycin was ordered for these patients but never documented as given in the EMR. The study population was predominately male with an average age of 55.7 years. Remaining subject demographics are summarized in Table 1. For secondary outcomes, the AUC/MIC group included 127 patients; the trough-based group included 137 patients (Table 2). Most patients were treated for skin and soft tissue infections (Table 1).
Table 1. Baseline Characteristics
| Parameter | All patients |
|---|---|
| Male, n(%) | 142 (53.8%) |
| Mean Weight, kg (SD) | 88.1 (26.7) |
| Mean BMIa, kg/m2 (SD) | 29.9 (8.9) |
| Mean Age, years (SD) | 55.7 (18.9) |
| Mean Scr, mg/dL (SD) | 1.1 (0.4) |
| Indications, n(%) | |
| Skin and Soft Tissue | 97 (36.7%) |
| Sepsis | 69 (26.1%) |
| Otherb | 63 (23.9%) |
| Pneumonia | 52 (19.7%) |
| Bacteremia | 23 (8.7%) |
aBody Mass Index
bIncludes urinary tract infection, osteomyelitis, and intra-abdominal infections
Table 2. Secondary endpoints
| Endpoint | AUC/MIC recommended dosing (n=127) | Trough-based dosing (n=137) | Difference [95% CI] | p-value |
|---|---|---|---|---|
| Estimated AUC/MIC, mg*hr/L | 510.9 SD:54.6 | 639.4 SD:136.7 | 128.5 [102.9:154.1] | p<0.001 |
| Estimated Trough, mg/dL | 13.5 SD:2.3 | 18.3 SD:5.0 | 4.8 [3.9:5.8] | p<0.001 |
| AKI, n (%) | 10 (7.9) | 11 (8.0) | p=0.9629 |
For the primary endpoint, mean total first day vancomycin dose was significantly higher (2649.6 mg; SD 66.6 mg) than the AUC/MIC recommended dosing (2380.7 mg; SD 831.8 mg) [95%CI 114.7:423.1] p=0.0007.
Once patients were divided into groups based on their dosing consistency with the AUC/MIC calculator recommendations, predicted mean AUC/MIC and trough concentrations were calculated and found to be significantly lower in the AUC/MIC group (510.9 [SD 54.6], 13.5 mg/dL [SD 2.3]) than in the trough-based group (639.4 [SD 136.7], 18.3 mg/dL [SD 5.0]) [95%CI 102.9:154.1, 95%CI 3.9:5.8] both p values<0.001 (Table 2).
Rates of acute kidney injury were similar between groups. Ten patients in the AUC/MIC group experienced an AKI compared to 11 in the trough-based group (Table 2). Of the 21 total patients who experienced an AKI, 17 (81%) were receiving at least one concomitant nephrotoxin. Overall, the incidence of AKI was 8%. Nephrotoxins included piperacillin/tazobactam (n=13), ACEi/ARB (n=6), IV contrast dye (n=5), and loop diuretics (n=4). Rates of other adverse events were low; two patients had allergic reactions to vancomycin and one patient developed Red Man syndrome. No other drug related adverse effects were reported.
DISCUSSION
The results of our study found that traditional trough-based dosing led to an increased total first day vancomycin dose compared to AUC/MIC recommended dosing. AUC/MIC recommended dosing also resulted in lower predicted AUC/MIC and trough concentrations. Our results support the guideline recommendations set forth in the 2020 IDSA guidelines of a target range of 400-600.1 These results were also described by Covvey et al., in their analysis of total daily dose of vancomycin in patients with MRSA bacteremia and a body mass index (BMI) greater than 30.13 Notably, the predicted trough concentration in the AUC/MIC group was 13.5 mg/dL which was almost 5 mg/dL lower than the mean trough-based concentration of 18.3 mg/dL; this depicts the ability to achieve AUC/MIC targets without trough concentrations of 15-20 mg/dL.2 It should be noted that total weight-based first day vancomycin dose was not calculated in this analysis, which may confound results as patients with lower weights may have reduced lower doses.
Lodise and colleagues explained the existence of an exposure-response relationship between initial vancomycin trough value and the occurrence of nephrotoxicity, as nephrotoxicity significantly increased with increasing initial trough concentration.8 Based on this relationship, lowering initial vancomycin exposure via utilizing AUC/MIC dosing recommendations may lower risks for nephrotoxicity that accompany elevated initial trough concentrations. A prospective study by Neely and colleagues utilized Bayesian estimations to calculate AUC/MIC and discovered AUC-guided dosing was associated with decreased nephrotoxicity.14 Additionally, Finch et al., found that AUC-guided dosing is independently associated with less nephrotoxicity and trough-concentrations, which they hypothesized is likely due to decreased vancomycin exposure.15 The results of our current study agree with their findings.
There are multiple potential obstacles to overcome when considering transitioning to an AUC/MIC-based dosing strategy, including increased workload on clinical pharmacists, the need for extensive education for multiple members of the healthcare team, and determining inclusion criteria for the new dosing strategy. Heilet al. provides advice for overcoming these obstacles including ideas for continued education for pharmacists, physicians, nurses, and phlebotomists.11 Additionally, new and innovative programs to calculate AUC/MIC exist which alleviate the excess workload that long-hand calculations place on clinical pharmacists. These programs include electronic calculators, such as what was utilized in this study, spreadsheets, calculators built into electronic medical records, and commercially available dosing calculators.11,12
Rates of acute kidney injury were similar between the two groups, although this study was not adequately powered to find differences in safety endpoints. The incidence of kidney injury was 8% which is consistent with rates reported in a recent meta-analysis.5 Additionally, most patients in this study were receiving concomitant nephrotoxins in addition to vancomycin. The effects of these nephrotoxins could not be adequately described in this evaluation, which is a limitation.
Furthermore, most patients in this study were treated for skin and soft tissue infections; the trough-based target for these infections is 10-15 mg/dL which may have induced bias by lowering the true vancomycin exposure in the trough-based group. Skin and soft tissue infections are also not considered invasive infections and it is currently unknown whether AUC/MIC based dosing is the best form of monitoring.1