INTRODUCTION
The Unified Health System (SUS) is the Brazilian Public National Health System funded by citizens' tax since 1988.1,2 SUS is responsible for supplying integral therapeutic care, including pharmaceutical services for all citizens from Brazil. Pharmaceutical services are related to the supply of drugs and their rational use, being the pharmacist a key role through pharmaceutical management and pharmaceutical care.2,3
Pharmaceutical management includes actions of selection, demand schedule, acquisition, storage, distribution, and dispensation of drugs. It is funded and organized through three components, being: 1) basic component (responsible for drugs of primary care plus health woman drugs and diabetes inputs); 2) strategic component (for smoking, food and nutrition, blood and hemoderivates, antiretroviral therapy, immunizing, and treatment of relevant epidemiology conditions for Brazil, as focal endemics); and 3) specialized component (for complex and high-cost treatment diseases).2
In Brazil, studies have reported problems in pharmaceutical management into the specialized component as a result of lack of planning and organization, which compromise the treatment of patients.4,5 On another hand, an effort has been made to improve the quality of pharmaceutical services, including the expansion of drugs offer.6
In 2009, the Minas Gerais State, one out of 27 Brazilian states, reorganized the public pharmacies investing in four axes: a) human resources: improving the pharmacists' salaries and contract technical professionals; b) physical structure: construction of new pharmacies with better installations and pharmacist' office; c) information: development of information systems to manage pharmaceutical services; d) education: improve the qualification of pharmacists and other professionals of pharmacies. This reorganized was called "Rede Farmácia de Minas", a network of community public pharmacies placed in the municipalities of the State.7-8 Thus, pharmaceutical services provided in Minas Gerais State differ from the other States of Brazil.
Among these pharmacies, 28 attend the specialized component to supply high-cost drugs, such as biological drugs used in the treatment of psoriatic arthritis (PsA). These pharmacies cover a health administrative region, which comprises a list of municipalities and a defined population.6
PsA is a chronic immune-mediated inflammatory disease characterized by peripheral arthritis, enthesitis, dactylitis, as well as axial, skin, and nail involvement.9-10 PsA must be properly diagnosed and treated to help minimize its impacts on patients' health.10-11
PsA patients present decreased quality of life, functional impairment, psychosocial disability, and significantly increased mortality rate in comparison to the overall population.12-15 The impact on their quality of life is directly related to the physical signs and symptoms of the disease, as well as to emotional and social aspects.15
Pharmacological treatment of PsA comprises glucocorticoids, non-steroidal anti-inflammatory drugs (NSAID), conventional synthetic, biological, and target synthetic disease-modifying antirheumatic drugs (DMARDs).16 Tumor necrosis factor inhibitors (anti-TNF) were the first biological DMARDs class available in the market and they play a key role in PsA treatment since they regulate inflammatory processes and inhibit progressive structural damage associated with this disease, improving the quality of life of the patients. However, approximately 50% of patients who take these drugs have difficulties in achieving adequate disease control.17 Also, these drugs are expensive and correspond to 90% of the total cost of PsA treatment.18
Currently, there are five approved anti-TNFs: (1) infliximab, a chimeric IgG anti-human monoclonal; (2) etanercept, a TNFR2 dimeric fusion protein, with an IgG1 Fc; (3) adalimumab, a fully human monoclonal antibody (mAb); (4) golimumab, also a fully human mAb and (5) certolizumab, a PEGylated Fab fragment. Infliximab is available in intravenous infusion and shall be administered in an adequate place like a hospital or a specialty clinic in biological infusion, while other anti-TNFs are available as subcutaneous injections and can be self-administered.19-20
Medication adherence is essential to enable adequate disease control and improve patients' quality of life.21 On the other hand, lack of adherence can compromise treatment outcomes.22 Medication adherence is defined as the extent to which the patient follows medical instructions, and it is overall described as Medication Possession Ratio (MPR) or Proportion of Days Covered (PDC).23-25 Medication persistence is defined as the time from the beginning of therapy until its discontinuation.25-26 Non-adherence and non-persistence lead to suboptimal clinical and humanistic outcomes and substantially burdens health systems; this process can significantly affect patients and health care providers.27 In this sense, this study aims to evaluate the medication adherence and persistence of PsA patients treated with anti-TNF drugs and verify its associated factors.
METHODS
Study type and population
A prospective observational study was performed at a single-specialty pharmacy in Belo Horizonte, the capital of the State of Minas Gerais, Brazil. This center is the biggest one out of 28 regional pharmacies from Rede Farmácia de Minas and assists about 320 PsA patients from 39 municipalities. The study period was from October 2011 to July 2019.
Patients 18 years of age or older, who were diagnosed with PsA - based on the Classification Criteria for Psoriatic Arthritis (CASPAR) and treated with adalimumab, etanercept, and infliximab were included in the study.28 Adalimumab and etanercept are subcutaneous drugs, while infliximab is an intravenous drug. Golimumab was excluded from the analyses because it was taken by a small number of patients. Also, it was only incorporated by SUS in 2017. Thus, PsA patients were exposed to adalimumab, etanercept, or infliximab use.
The sample size was calculated considering the binary outcome (adherence/non-adherence and persistent/non-persistent). A difference of 30% in the proportion among groups (difference=0.30), a statistical significance of 5% (alpha=0.05), and a power test of 80% (beta=0.80) were used, which indicated a minimal sample of 27 patients per group, in a total of 81 patients.
Data collection
Patients with a prescription for use of biological therapy request the drug to the State, which evaluated the documentation (form and exams) and authorized the treatment. After the authorization, patients were invited by telephone call to participate in the study, and the first drug dispensation was scheduled. Patients were attended to monthly or bimonthly to receive their drugs and orientations, and an information system was used to record and manage all patient treatments.
Two pharmacy information systems were used for data collection due to system change during the study period. These systems record the dispensing of drugs, including the type and amount dispensed for each patient. Thus, it was possible to assess patients' adherence and persistence for 52 weeks. Biological drug switches, absence of treatment renewals every three months, and death were considered discontinuation of treatment.
Interviews were conducted with patients in the first drug dispensation based on a standardized form to collect baseline data. A research team composed of pharmacists, undergraduate, and graduate pharmacy students conducted the interviews. Participants' sociodemographic data, disease activity scores by the Clinical Disease Activity Index (CDAI) and Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), as well as functionality by Health Assessment Questionnaire (HAQ) and quality of life by European Quality of Life Five-dimensions (EQ-5D), were collected to characterize the patients and evaluate the predictors of medication adherence and persistence.29 CDAI, BASDAI, HAQ, and EQ-5D were also collected at 12 months for adherence and persistent patients to evaluate the treatment effectiveness.
Outcomes
CDAI is a peripheral disease activity measure used in rheumatic diseases that present a good correlation with specific instruments for PsA later developed. CDAI values can range from 0 to 76. High disease activity is defined as a CDAI >22, moderate activity as a CDAI >10 and ≤22, low activity as a CDAI ≤10 and >2.8, and remission as a CDAI ≤2.8. BASDAI is an axial disease activity measure used in spondylarthritis and recommended by the guideline of PsA in Brazil until 2018 when it was replaced by ASDAS (Ankylosing Spondylitis Disease Activity Score). BASDAI scores range from 0 (best) to 10 (worst). A score >4 is accepted to indicate active disease. HAQ-DI and EQ-5D both validated measures for Brazil are used to evaluate the functional status and quality of life, respectively. HAQ scores from 0 to 1 are generally considered to represent mild to moderate difficulty, 1 to 2 moderate to severe disability, and 2 to 3 severe to very severe disability. EQ-5D is a generic instrument used mainly to obtain data of utility for economic models or to compare different populations. These outcomes have been used in PsA as effectiveness measures in observational studies.29
Medication adherence was assessed through indirect PDC measurement, which was calculated by the ratio of the number of days the patient is covered by the medication to the number of days the patient is eligible to have the medication on hand.30
PDC was calculated by following the steps below:
1) The analysis period was 52 weeks, which corresponds to 12 months of follow-up.
2) The number of doses dispensed to each patient in the period was summed based on data provided by the information systems.
3) The ideal amount of drug dispensed was calculated for each drug. According to the Brazilian Clinical Protocol and Therapeutic Guideline of PsA, etanercept was administered every week, adalimumab every two weeks, and infliximab at 0, 2, and 6 weeks (induction dose); and then, every 8 weeks (maintenance dose). Therefore, the ideal number of drugs to be dispensed within 52 weeks is 26 doses to adalimumab, 52 doses to etanercept, and 7 doses to infliximab.31
4) Finally, the number of doses dispensed within 52 weeks for each patient was divided by the ideal number of doses needed to cover patients' treatment throughout the study period.
Patients with PDC ≥0.8 were classified as adherent to the anti-TNF drug since this value has been adopted as a cutoff point for rheumatic diseases.32,33
Medication persistence was defined as the duration time from the beginning of the therapy to its discontinuation. Treatment discontinuation (non-persistence) was defined as lack of drug dispensing after 90 days from the last dispensing date - this period corresponds to treatment renewal at SUS. The patient must renew his solicitation every three months through a medical prescription and a drug solicitation form.18,34,35 Then it was possible to evaluate the proportion of persistent individuals for each anti-TNF drug at 12-month follow-up.
The time elapsed until treatment discontinuation was calculated as the time between the first and the last drug dispensing, plus 30-day grace period (drug possession), except for infliximab in the maintenance phase, which was given a 60-day grace period. The grace period was defined based on the treatment coverage period from the drug dispensing day. These definitions have been used to evaluate the medication persistence for other rheumatic diseases in Brazil.18,34,35
Statistical analysis
Descriptive data analysis was performed through mean and standard deviation for continuous variables and frequency measures for categorical variables. Pearson's Chi-square test was used to analyze differences among groups for categorical variables. Independent t-test was used to analyze the differences between 2 groups (adherent and non-adherent patients), paired t-test to verify the difference between baseline and 12 months, and ANOVA with Bonferroni adjustment for differences among 3 groups (adalimumab, etanercept, and infliximab) for continuous variables. Missing clinical data at 12 months were handled through multiple imputations by chained equations using a predictive mean matching with three nearest neighbors.36
Medication persistence in PsA patients attended by the Belo Horizonte specialty pharmacy was compared with the national PsA population attended in the SUS specialized component in Brazil. Da Silva and collaborators (2019) used the same definition of medication persistence applied to a national database of SUS.18
Kaplan-Meier survival curves were outlined to verify the time until treatment discontinuation at 12 months. The log-rank test was used to investigate the differences among drugs for the medication persistence outcome.
Log-binomial regression analysis was used to investigate associated factors with non-adherence and non-persistence to the anti-TNF drugs. Patients' sociodemographic and clinical features collected at baseline were used as independent variables, including the disease activity measure by CDAI and BASDAI. Variables presenting p-value<0.20 in the bivariate analysis were included in the multivariate analysis. Variables presenting p<0.05 remained in the final model.
Data analysis was carried out in STATA software, version 16.1.
RESULTS
One hundred ninety-seven PsA patients were included in the study. The mean age was 50.8 years (11.3). Most patients were women (57.4%), white (52.3%), married (58.5%), with complete high school education (40.0%), and had at least one comorbidity (74.1%) (Table 1).
Table 1. Sociodemographic and clinical characteristics of PsA patients treated with anti-TNF drugs at Belo Horizonte Specialty Pharmacy
| Variables | Total (197) |
|---|---|
| Sex - n (%) | |
| Female | 113 (57.4) |
| Male | 84 (42.6) |
| Age in years - mean (SD) | 50.83 (SD=11.27) |
| Disease time - mean (SD) | 5.96 (SD=7.51) |
| Ethnicity n (%) | |
| White | 103 (52.3) |
| Brown | 66 (33.5) |
| Others | 28 (14.2) |
| Marital Status n (%) | |
| Single | 49 (25.1) |
| Married | 114 (58.5) |
| Others | 31 (16.4) |
| Schooling n (%) | |
| Illiterate until complete elementary school | 55 (28.2) |
| High school | 78 (40.0) |
| Higher education | 62 (31.8) |
| Anti-TNF n (%) | |
| Adalimumab | 112 (56.8) |
| Etanercept | 64 (32.5) |
| Infliximab | 21 (10.7) |
| Current drugs n (%) | |
| csDMARDs | 86 (43.6) |
| NSAIDs | 48 (24.4) |
| Corticosteroids | 53 (26.9) |
| Previous drugs n (%) | |
| csDMARDs | 152 (78.4) |
| bDMARDs | 40 (20.5) |
| CDAI - mean (SD) | 22.58 (SD=16.79) |
| BASDAI - mean (SD) | 5.28 (SD=2.53) |
| HAQ-DI - mean (SD) | 1.21 (SD=0.71) |
| EQ-5D - mean (SD) | 0.65 (SD=0.18) |
| EQ-5D VAS - mean (SD) | 62.92 (SD=20.30) |
| Comorbidities n (%) | |
| 0 | 51 (25.9) |
| 1 | 55 (27.9) |
| > 1 | 91 (46.2) |
VAS: Visual Analogic Scale; BASDAI: Bath Ankylosing Spondylitis Activity Index; bDMARD: Biological disease-modifying antirheumatic drug; CDAI: Clinical Disease Activity Index; EQ-5D: EuroQol-5 dimensions; HAQ: Health assessment questionnaire; n: Number of patients; NSAIDs: Nonsteroidal anti-inflammatory drugs; SD: Standard deviation; csDMARD: Conventional synthetic disease-modifying antirheumatic drug.
Adalimumab (56.9%) was the most used anti-TNF drug. Concomitant synthetic drug use to PsA was observed in 43.7% of the patients using conventional synthetic DMARDs, 24.4% NSAIDs; and 26,9% glucocorticoids (Table 1). Patients presented a CDAI mean of 22.58 (SD=16.79), a BASDAI mean of 5.28 (SD=2.53), a HAQ mean of 1.21 (SD=0.71), and a utility score of 0.65 (SD=0.18) by EQ-5D. These values indicate a moderate to high peripheral (CDAI) and axial (BASDAI) disease activity, and a moderate functionality impairment (HAQ) for these patients.
The medication adherence to anti-TNF drugs was 74.6% at one year. Patients using infliximab presented the highest adherence rate (90.5%); it was followed by adalimumab (75.9%) and, finally by etanercept (67.2%). There was a statistically significant difference for infliximab versus etanercept (p=0.037) (Table 2).
Table 2. Medication adherence, persistence, and time until discontinuation of PsA patients treated with anti-TNF drugs
| Anti-TNF | Adherence; n (%) | Persistence; n (%) | Time until discontinuation; mean (SD) |
|---|---|---|---|
| Adalimumab (n =112) | 85 (75.9) | 82 (73.2) | 325.34 (SD=85.55) |
| Etanercept (n=64) | 43 (67.2) | 40 (62.5) | 302.81 (SD=104.77) |
| Infliximab (n=21) | 19 (90.5) | 20 (95.2) | 356.52 (SD=38.84) |
| Total | 147 (74.6) | 142 (72.1) | 321.35 (SD=89.89) |
| P-value1 | 0.212 | 0.138 | 0.323 |
| P-value2 | 0.137 | 0.028 | 0.426 |
| P-value3 | 0.037 | 0.004 | 0.052 |
n: Number of patients; SD: Standard deviation
p-value1: adalimumab vs etanercept; p-value2: adalimumab vs infliximab; p-value3: etanercept vs infliximab
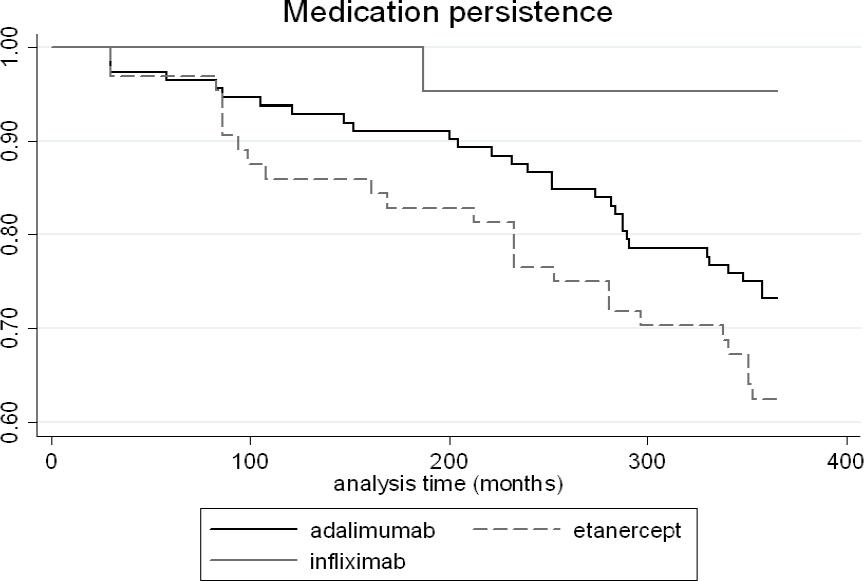
Persistent patients comprised 72.1% of the investigated population. There were differences among anti-TNF drugs (log-rank test: p=0.017) (Figure 1). Patients treated with infliximab also presented the highest persistence rate (95.2%) in comparison to patients treated with other drugs (p<0.05). There were no differences among drugs for the time until treatment discontinuation (Table 2). The main reasons for discontinuing to follow-up at 12 months were therapeutic failure (n=19; 9.6%), adverse reactions (n=12; 6.1%), impossibility of contact (n=10; 5.1%), withdraw consent (n=9; 4.6%), and impossibility to go to the pharmacy (n=5; 2.5%).
Medication persistence was higher for the patients treated with biological therapy by Belo Horizonte Specialty Pharmacy than overall PsA patients in Brazil (72.1% versus 56.5%; p<0.001). Patients using adalimumab (73.2% versus 59.3%; p=0.001) and infliximab (95.2% versus 45.3%; p<0.001) at Specialty Pharmacy also achieved higher medication persistence than overall PsA patients from SUS in Brazil (Figure 2).
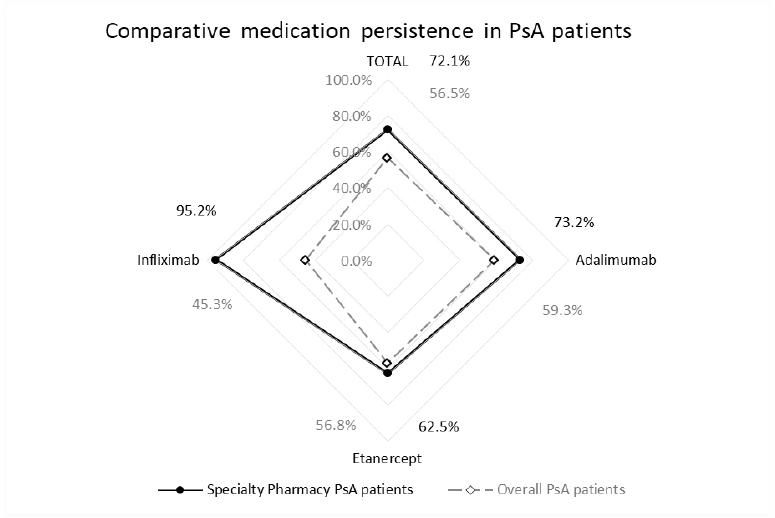
Figure 2. Comparative medication persistence of PsA patients treated with anti-TNF drugs at Belo Horizonte specialty pharmacy and overall ones
Clinical missing data were observed in 25.4% of the persistent patients and 28.9% of the adherence patients. All clinical measures had a significant improvement at 12 months, which indicates the effectiveness of the treatment (p<0.05) (Table 3). No differences were observed at baseline and in the effectiveness at 12 months among drugs (p>0.05).
Table 3. Effectiveness, functionality, and quality of life of PsA patients treated with biological therapy at 12 months
| Adherence patients (n = 147) | Persistents patients (n=142) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anti-TNF drug | Baseline | 12 months | Diff. | p-value | Anti-TNF drug | Baseline | 12 months | Diff. | p-value | ||||
| mean | SD | mean | SD | mean | SD | mean | SD | ||||||
| CDAI | CDAI | ||||||||||||
| All Anti-TNF | 21.86 | 17.19 | 11.51 | 12.65 | -10.34 | <0.001 | All Anti-TNF | 21.85 | 16.87 | 10.92 | 12.32 | -10.93 | <0.001 |
| Adalimumab | 21.72 | 17.42 | 10.29 | 11.44 | -11.43 | <0.001 | Adalimumab | 22.06 | 17.07 | 10.31 | 11.76 | -11.74 | <0.001 |
| Etanercept | 20.98 | 16.69 | 12.04 | 13.21 | -8.94 | <0.001 | Etanercept | 20.12 | 16.43 | 9.86 | 11.49 | -10.26 | 0.001 |
| Infliximab | 24.43 | 17.98 | 15.66 | 15.85 | -8.77 | 0.011 | Infliximab | 24.52 | 17.36 | 15.46 | 15.43 | -9.06 | 0.008 |
| BASDAI | BASDAI | ||||||||||||
| All Anti-TNF | 5.00 | 2.62 | 2.98 | 2.29 | -2.02 | <0.001 | All Anti-TNF | 4.98 | 2.58 | 2.88 | 2.29 | -2.10 | <0.001 |
| Adalimumab | 4.78 | 2.53 | 2.81 | 2.20 | -1.96 | <0.001 | Adalimumab | 4.80 | 2.52 | 2.77 | 2.26 | -2.03 | <0.001 |
| Etanercept | 5.09 | 2.63 | 2.91 | 2.11 | -2.19 | <0.001 | Etanercept | 4.97 | 2.55 | 2.62 | 1.95 | -2.34 | <0.001 |
| Infliximab | 5.74 | 2.98 | 3.86 | 2.94 | -1.88 | <0.001 | Infliximab | 5.78 | 2.88 | 3.85 | 2.84 | -1.93 | <0.001 |
| Functionality (HAQ) | Functionality (HAQ) | ||||||||||||
| All Anti-TNF | 1.14 | 0.70 | 0.77 | 0.62 | -0.36 | < 0.001 | All Anti-TNF | 1.13 | 0.70 | 0.74 | 0.61 | -0.39 | 0.001 |
| Adalimumab | 1.10 | 0.70 | 0.71 | 0.57 | -0.39 | < 0.001 | Adalimumab | 1.12 | 0.71 | 0.68 | 0.58 | -0.44 | <0.001 |
| Etanercept | 1.17 | 0.74 | 0.87 | 0.65 | -0.30 | 0.003 | Etanercept | 1.11 | 0.72 | 0.78 | 0.64 | -0.32 | <0.001 |
| Infliximab | 1.24 | 0.66 | 0.84 | 0.74 | -0.40 | 0.003 | Infliximab | 1.21 | 0.67 | 0.87 | 0.70 | -0.34 | 0.005 |
| Quality of Life (EQ-5D) | Quality of Life (EQ-5D) | ||||||||||||
| All Anti-TNF | 0.67 | 0.19 | 0.76 | 0.17 | 0.09 | <0.001 | All Anti-TNF | 0.66 | 0.18 | 0.76 | 0.17 | 0.10 | <0.001 |
| Adalimumab | 0.66 | 0.18 | 0.77 | 0.16 | 0.10 | <0.001 | Adalimumab | 0.65 | 0.18 | 0.77 | 0.17 | 0.11 | <0.001 |
| Etanercept | 0.68 | 0.18 | 0.78 | 0.14 | 0.10 | <0.001 | Etanercept | 0.67 | 0.18 | 0.79 | 0.13 | 0.12 | <0.001 |
| Infliximab | 0.64 | 0.22 | 0.68 | 0.23 | 0.04 | 0.177 | Infliximab | 0.66 | 0.22 | 0.69 | 0.23 | 0.03 | 0.268 |
Diff: difference; SD: Standard deviation
The associated factors to higher medication adherence were lower BASDAI (p<0.001), non-white race (black, brown, and other) (p=0.005), and intravenous drug use (p=0.002). The associated factors to higher medication persistence were lower BASDAI (p=0.001), intravenous drug use (p<0.001), non-use of corticoids and NSAIDs (p=0.006; p=0.011) and has comorbidity (p=0.009) (Table 4).
Table 4. Predictors of medication adherence and persistence in PsA patients treated with anti-TNF drugs at Belo Horizonte Specialty Pharmacy (multivariable analysis)
| Medication adherence | Medication persistence | ||||
|---|---|---|---|---|---|
| Variables | RR [95%CI] | p-value | Variables | RR [95%CI] | p-value |
| BASDAI | 0.94 [95%CI 0.91:0.97] | <0.001 | BASDAI | 0.94 [95%CI 0.90:0.97] | 0.001 |
| Ethnicity | 0.005 | NSAID [no use] | 1.30 [95%CI 1.06:1.60] | 0.011 | |
| White | 1 | Comorbidities [yes] | 1.34 [95%CI 1.08:1.66] | 0.009 | |
| Non-white | 1.28 [95%CI 1.08:1.51] | Corticoids [no use] | 1.37 [95%CI 1.09:1.71] | 0.006 | |
| bDMARD | 0.002 | bDMARD | <0.001 | ||
| Subcutaneous | 1 | Subcutaneous | 1 | ||
| Intravenous | 1.36 [95%CI 1.12:1.66] | Intravenous | 1.58 [95%CI 1.30:1.91] | ||
BASDAI: Bath Ankylosing Spondylitis Activity Index; bDMARD: biological disease-modifying antirheumatic drug; RR: risk ratio; CI: confidence interval
DISCUSSION
The present study assessed the medication adherence and persistence, beyond the associated factors to these outcomes in PsA patients treated with anti-TNF-drugs. In this regard, few studies have been evaluated medication adherence and persistence in PsA patients.18,37-40
The profile of sociodemographic and clinical characteristics for PsA patients was like other studies, including mean age, comorbidities, ethnicity, schooling, as well as biological DMARDs, conventional synthetic DMARDs, NSAIDs, and glucocorticoids use.17,39-41
Adherence to anti-TNF drugs was observed for 74.6% of patients. Oelke and collaborators (2019) reported an overall adherence rate of 40.4% in PsA patients using biological therapy in the United State of American, lesser than observed in our study.37 Santoleri et al. (2020) reported an overall medication adherence rate of 83% for anti-TNF drugs in a hospital setting, which indicates superior adherence to an outpatient setting.38 Murage and collaborators (2018) carried out a systematic review to evaluate medication adherence for rheumatoid arthritis, psoriasis, and psoriatic arthritis and they did not find studies that evaluate medication adherence rates for PsA patients.21
Adherence to adalimumab varies from 42.6% in an outpatient setting to 83.0% in a hospital setting. Adherence to etanercept varied from 47.7% in an outpatient setting to 84.0% in a hospital setting.37,38 Data on medication adherence with infliximab was not found.
Medication persistence to anti-TNF drugs was 72.1%, being highest for infliximab (95.2%), followed by adalimumab (73.2%) and etanercept (62.5%). The literature has reported medication persistence of 61.0% to biological therapy in PsA patients being 74% for infliximab, 56% for adalimumab, and 51% for etanercept at one year.21 In a national database analysis in Brazil, which used the same definition and methodology, medication persistence found to anti-TNF therapy was 56.5%.18 Therefore, the medication persistence observed in our study is higher than reported by other studies, including Brazil. It is an important finding once medication persistence has been used as a proxy of effectiveness and safety for biological therapies applied to rheumatic diseases.18,34,35
Intravenous administration route and lower BASDAI were identified as associated factors to medication adherence and persistence. Bolge et al. (2017) reported that patients using intravenous biologics are highly satisfied with their medications and perceive the opportunity for health care provider interaction at their infusion facilities as an advantage of their regimen.42 Besides, a poor clinical status at baseline to be a predictor of non-adherence and non-persistence in rheumatic diseases.43
Other associated factors were a non-white race to medication adherence, and no use of NSAIDs and glucocorticoids, besides the presence of comorbidity to medication persistence. The non-white race was reported as an associated factor to medication adherence in a systematic review.21 NSAIDs and glucocorticoids are used to control symptoms of active disease for a brief time and the absence of its use indicates good control of disease with biological therapy, which contributes to medication adherence and persistence. Also, glucocorticoids can be challenging in PsA because of the risk for the flare of psoriasis upon withdrawal.44 At last, generally, the presence of comorbidities is associated with non-persistence.18,21
Clinical outcomes, functionality, and quality of life were improved in patients with medication adherence and persistence, which corroborates the effectiveness of biological therapy of other studies.17,41,45,46 Thus, adequate disease management is fundamental to medication adherence and persistence.
Medication adherence and persistence were higher than most studies found, including in Brazil. These results are very important since point to the benefits of pharmaceutical services. The Rede Farmácia de Minas, which the specialty pharmacy of this study is part has been considered a cost-effective model to provide pharmaceutical services.7 Medication adherence also contributes to effectiveness, safety, and helps avoid additional health care costs.47
Despite progress in pharmaceutical management in Brazil, pharmaceutical care services still are incipient.48 Studies on other conditions have reported that pharmaceutical care services improve medication adherence, clinical outcomes and reduce hospitalizations.49,50 Therefore, the implementation of pharmaceutical care services in the Rede Farmácia de Minas is needed to complete the structuration process started in 2009.
Medication adherence and persistence are fundamentals to achieve therapeutic goals but studies that evaluated and propose strategies to improve these outcomes for rheumatic diseases are scarce.51 Also, a better experience for patients is in need and some strategies can be used to maintain and improve medication adherence as patient education, health literacy, besides pharmaceutical care services. Pharmacists are often involved in these actions contributing to pharmacotherapy, working together with other health professionals and patients. Thus, to assess adherence and offer advice to physicians about simplifying and improving drug regimens, and direct counseling of patients by pharmacists may be particularly promising because of pharmacists' specialized training, and knowledge of medications and availability to patients.52,53
This study has strengths and limitations. This study is original in evaluating medication adherence for PsA patients. Also, few studies have reported medication persistence outcomes. Thus, the results fill a gap in knowledge for PsA. In this sense, real-world evidence is useful to complement current knowledge about biological therapy in PsA. Finally, medication persistence has been adopted as a proxy for effectiveness and safety in biological therapy for immunomodulated diseases and high medication persistence was observed in our study, which indirectly indicates the benefits of biological therapy.
As limitations can be cited convenience sample used, that is, only patients who were attended by the pharmacy and agreed to participate in this study were included. Thus, patients with a poor prognosis may not have participated because it was not possible to attend the pharmacy to seek their drug. Also, the number of patients that decline to participate in the study was not registered. Finally, PDC is an indirect method based on the drug supply and no guarantee of drug use by patients can be made. Therefore, the results shall be interpreted and generalized with caution.
CONCLUSIONS
Patients with medication adherence and persistence had significant improvements in clinical measures, functionality, and quality of life. High medication adherence and persistence to biological therapy were observed and associated with lesser disease activity at baseline. Also, medication persistence to PsA patients attended in specialty pharmacy was higher than overall PsA population attended by specialized component in Brazil, which indicates the importance of pharmaceutical services to provide health care and promote the effectiveness and safety of biological therapies.