Neuropsychiatric symptoms (NPS) constitute a psychopathological dimension of neurodegenerative diseases consisting of changes in personality and the presence of behavioural disorders (e.g. increased social disinhibition, impulsivity or irritability), or alterations in basic functions such as appetite or sleep among others (Masters et al., 2015; Teng et al., 2007; Acosta-Castillo, 2012; Lanctôt et al., 2017; Ballard et al., 2008). NPS have a high prevalence in dementias reaching rates of up to 90% (Nowrangi et al., 2015; Aarsland et al., 2007) and 59% for Mild Cognitive Impairment (MCI) (Feldman et al., 2004). NPS are associated with a higher burden of pathological markers of dementia (Zubenko et al., 1991), caregiver stress (Fischer et al., 2012a), faster conversion from MCI to Dementia (Landau et al., 2010; Rosenberg et al., 2013), functional impairment (Fischer et al., 2012b) as well as higher mortality rates (Lanctôt et al., 2017) and poorer quality of life (Karttunen et al., 2011). Furthermore, these alterations can occur in preclinical and prodromal stages of neurodegenerative pathologies (Steinberg et al., 2008). In turn, the presence of NPS is a risk factor in a cognitively healthy population for the subsequent development of cognitive impairment (Burhanullah et al., 2020; Wise et al., 2019), as shown by several studies such as the Alzheimer's Disease Cooperative Study (Banks et al.,2014 ), the Alzheimer's Disease Research Center (Donovan et al., 2014), the Danish Psychiatric and Medical Register (Cerejeira et al., 2012), the Medical Research Council Cognitive Function and Ageing Study (Köhler et al., 2013) or the National Alzheimer's Coordinating Center Study (Masters et al., 2015; Leoutsakos et al., 2015).
Nowadays, correct identification of these NPS is important to avoid psychiatric diagnoses in the elderly population, in the absence of cognitive impairment, without considering the possibility of neurodegenerative pathology and, therefore, offering inappropriate or delayed care (Woolley et al., 2011). Diagnosis of these symptoms is usually based on expert opinion and experience in the field, as opposed to standardised tests (Cummings, 2021). However, different scales have been developed to assess NPS. Among others, the Behavioural Pathology Assessment Scale for Alzheimer's Disease (BEHAVE-AD) (Banks et al., 2014), and the Neuropsychiatric Inventory (NPI) (Cummings et al., 1994), developed for the diagnosis of NPS in dementia. More recently, the Mild Behavioral Impairment Checklist (MBI-C) (Ismail et al., 2017) has been published, based on the criteria established in 2016 by the International Society to Advance Alzheimer's Research and Treatment (ISTAART) for the diagnosis of Mild Behavioural Impairment (MBI) (Ismail et al., 2016) (see Table 1). The aim of the MBI-C is to assess NPS as markers of prodromal and preclinical stages of neurodegenerative diseases and not in stages of dementia like the previous ones. However, the 34 items of the test make it difficult to use in Primary Care consultations as a gateway to the Public Health System, and a shorter, simpler screening test is needed. In turn, there is often a discrepancy between the NPS reported by the patient and those provided by the family member or caregiver (Chen et al., 2022), and it could be very useful for both family members and caregivers to have a quick way to report these behaviours, without having to make a direct comparison with the information provided by the patient himself/herself.
Table 1. Criteria of the International Society to Advance Alzheimer's Research and Treatment for the Mild Behavioral Impairment Modified fromAguera-Ortiz (2017)
| Changes in personality or behaviour observed by the patient, informant family member or health professional, with onset late in life (over 50 years of age) and which persist for a period of six months or more, at least intermittently. This condition is a clear change from the person's usual personality or behaviours, and is evident in at least one of the following aspects: a) Decreased motivation (e.g. apathy, loss of spontaneity or indifference). (b) Affective dysregulation (e.g. anxiety, dysphoria, emotional lability, euphoria or irritability) (c) Loss of impulse control (e.g. agitation, disinhibition, pathological gambling, obsessiveness, perseverative behaviours, excessive attachment to certain stimuli) (d) Social inappropriateness (e.g. lack of empathy, loss of insight, loss of social skills or tact, psychic rigidity or exaggeration of previous personality traits) (e) Abnormal perceptions or altered thought content (e.g. hallucinations or delusions). |
| 2. Behaviours are of sufficient severity to result in at least minimal dysfunction in at least one of the following areas: (a) Interpersonal relationships (b) Other aspects of social functioning c) Ability to function in the workplace. The patient should generally maintain independent functioning in daily life, with minimal help or assistance. |
| 3. Although comorbid conditions may be present, the personality or behavioural changes are not attributable to another current psychiatric disorder (e.g. generalised anxiety disorder, major depression, manic or psychotic disorders) or to a medical, traumatic or physiological origin due to the physiological effects of a medication or substance. |
| 4. Patients do not meet criteria for a dementia syndrome (e.g. Alzheimer's disease, frontotemporal dementia, dementia with Lewy bodies, vascular dementia or other dementias) Mild behavioural impairment may be diagnosed concurrently with mild cognitive impairment. |
Therefore, the aim of this study is the construction and validation of a screening test for relatives and caregivers for the identification of NPS associated with neurodegenerative pathologies in preclinical and prodromal stages of the disease, based, like the MBI-C, on the criteria proposed by the ISTAART for the diagnosis of MBI.
Method
Participants
The total study sample consisted of 206 participants (63 men and 143 women) over 55 years of age (mean 77 years and standard deviation 10.58), mostly with basic education (135 basic studies, 47 medium and 24 higher) and resident in the Principality of Asturias (Spain). The sample was obtained in several Day-care Centres, nursing homes and health centres where professionals informed users and patients who met the inclusion and exclusion criteria about the possibility of participating in the study, and if they did, they were quoted. All subjects were selected by non-probabilistic sampling, had to have Spanish as their mother lenguage and had to have a family member or professional caregiver as an informant. As exclusion criteria, subjects could not present a psychiatric diagnosis, acquired brain damage, metabolic alterations or a diagnosis of dementia. Of the participants in the study, 117 had no previous neurological diagnosis and 89 had a diagnosis of MCI, of which 45 had no specific pathology affiliation, 26 associated with AD, 4 associated with vascular alteration, 11 with Idiopathic Parkinson's Disease, 1 associated with Degeneration with Lewy Bodies and 2 associated with Frontotemporal Lobar Degeneration.
Instruments
Among the instruments used in the study we can differentiate between those used for the selection of participants, assessing overall cognitive and functional performance, and those used for the validation of the Oviedo Questionnaire of Mild Behavioural Impairment (Cuestionario Oviedo de Deterioro Conductual Leve, CO-DCoL).
The first were incorporated into decision-making on the possible clinical diagnosis of MCI, ruling out excessively low cognitive performance or very low functional capacity as they would indicate an impairment compatible with dementia criteria.
Among the tests used to assess the global cognitive performance of the subjects were the 7-Minute Test, which has a sensitivity of .92 for AD and .89 for other dementias, and a specificity of .93 (Meulen et al., 2004), the Frontal Assessment Battery (FAB), with an internal consistency αv= .60, an intraclass correlation of .72 and a test-retest reliability of .70 (Dubois et al., 2000) and the Minimental State Examination (MMSE) (Blesa et al., 2001), with a sensitivity of .85, a specificity of .90 and an intra-observer reliability of .93 (Lobo et al., 1999).
On the other hand, the Spanish version of the Lawton and Brody Index, specially designed to assess the Instrumental Activities of Daily Living (Lawton & Brody, 1969), was applied to assess the functionality of the subject, with an inter and intra-observer reliability of .94 (Ferrín et al., 2011), with a reliability of α = .93 in our sample of subjects.
With regard to the validation of the CO-DCoL, the 15-item version of the Yesavage Geriatric Depression Scale (GDS-15) (Yesavage et al., 1982) was used to assess the mood of the participant, with a sensitivity of .81 and a specificity of .97 (Ortega-Orcos et al., 2007). The reliability in the current sample was α =.70. For the NPS, the Neuropsychiatric Inventory (NPI) in its reduced version in Spanish (NPI-Q) was used, where sensitivity and specificity vary depending on the subtest, with values ranging from .88 for the hallucinations scale, .95 for depression and 1 for the others. Specificity ranges between .85 for apathy/indifference and 1 for hallucinations (Boada et al., 2002). In our sample, test-retest reliability was high with α =.86. The Mild Behavioral Impairment Checklist (MBI-C) (Aguera-Ortiz et al., 2017) was also used to assess NPS, with a sensitivity of 1 and a specificity of .78 (Mallo et al., 2018) and a reliability in the study sample of α =.90. Finally, together with the neuropsychiatric tests, the CO-DCoL was applied, which is presented in detail in the rest of the paper.
Procedure
For the sample collection, help was requested from the health centres, nusing homes and Day- care centres of the Principality of Asturias to inform and refer users to the study. This study was approved by the Research Ethics Committee, as well as by the Social Services Ethics Committee of the Principality of Asturias. Written informed consent was obtained from all participants prior to the study preserving anonymity in accordance with the LOPD GDD 3/2018, Law 14/2007 on biomedical research and Regulation (EU) 2016/679 of the European Parliament and of the Council.
The creation of the instrument was based on the 5 domains of the MBI as described in the ISTAART Criteria (Ismail et al., 2016). The first domain would be constituted by Diminished Motivation which would encompass symptoms such as apathy, loss of spontaneity or indifference; the second would be Affective Dysregulation where we find symptoms such as anxiety, dysphoria or emotional lability among others. The third domain would be Loss of Impulse Control (e.g. agitation, disinhibition, pathological gambling, obsessiveness, etc). The fourth domain is Social Inadequacy (e.g. lack of empathy, loss of insight, loss of social skills, etc.) and the fifth domain is Anomalous Perceptions or Alterations in Thought Content where hallucinations or delusions are included.
The aim was to create a comprehensive list of relevant and simple questions to assess NPS and then reduce it, using different statistical methods, to create a final version that can be applied in clinical practice quickly and reliably. To this end, a total of 50 initial items were constructed, related to the identification of NPS, on a 4-point Likert-type scale (0: No changes, 1: Slight changes, 2: Moderate changes, 3: Severe changes) which were sent to 10 experts in the field of cognitive and behavioural assessment of neurodegenerative diseases and who have years of experience in different centres for the assessment and diagnosis of this type of pathology, as well as university teaching experience in the area of neuropsychology. They were asked to report on the degree to which these impairments correctly represent the theoretical construct of the MBI. To this end, they were asked to score with "0" those items that did not seem to be representative of the MBI, in any of its domains, and with "1" those that did seem to be representative. In turn, they were asked to indicate those that seemed most appropriate, as well as to add other items or recommendations. Subsequently, by means of matching and taking a congruence index of 0.7 as a reference, 38 items were selected from the initial total to be applied to the study participants. Those that for various reasons had been indicated as being difficult for the subjects to understand were eliminated.
Relatives or profesional caregivers were assessed by clinical interview and using the instruments, described above, GDS-15, NPI-Q, MBI-C and CO-DCoL, the subjects participating in the study were assessed with MMSE, the 7-minute test and the FAB. The entire assessment was conducted in a single session of approximately one hour and fifteen minutes. Family members or caregivers were initially given an individual semi-structured clinical interview lasting 20-35 minutes, during which they were explained and asked to complete questionnaires to assess the functionality, emotional and neuropsychiatric disturbances they had observed in the participants they cared for. Subsequently, study participants underwent an individual cognitive and emotional assessment and were asked for socio-demographic data while family members completed the different questionnaires outside the consultation room. Where necessary, relatives were allowed to conduct the clinical interview by telephone and to complete the neuropsychiatric questionnaires telematically using a Google Forms questionnaire.
Finally, the diagnosis of MBI was made by means of a semi-structured interview together with the cognitive and functional assessment of the patient and the medical data provided by the participant or by the professionals of the health centres, nursing homes and Day-care centres where the study was carried out and in accordance with the ISTAART-AA criteria. For criteria one and two, the families were asked about the presence of NPS in the last six months, as well as about the possible appearance of alterations in activities of daily living and the Lawton and Brody Questionnaire (Lawton & Brody, 1969) was applied. For criterion three, the interview provided all the necessary information. Criterion four was obtained on the basis of the participant's cognitive assessment and the information provided in medical reports on previous diagnoses.
Data Analysis
The data were analysed using SPSS v.20 and Factor software (Ferrando & Lorenzo-Seva, 2017, Lorenzo-Seva & Ferrando, 2013). In order to select the most appropriate items, as well as to know the internal structure of the test, the total score of the items was calculated and an Exploratory Factor Analysis was performed using a Pearson Correlation Matrix with the extraction method of Unweighted Robust Least Squares and the procedure to determine the number of factors was Parallel Analysis. The statistical fit of the model was checked by means of the GFI, RMSEA, CFI and NNFI tests, taking values above .95 and below .06 in the case of the RMSEA as fit criteria, and the Unidimensional Congruence (UniCo), Explained Common Variance (ECV), and Mean of Item Residual Absolute Loadings (MIREAL) indices were used to study the adequacy of the data to a single dimension. The following values are taken as reference to treat the data as essentially one-dimensional: UniCo > .95; ECV > .85; MIREAL < .30 (Calderón-Garrido et al., 2019). Once the final items were selected, reliability estimation was carried out using Cronbach's Alpha and the Omega Coefficient. We studied whether the items presented impact and Differential Functioning (DIF) in relation to gender using the logistic regression procedure (Gómez-Benito et al., 2013). The convergent validity of the test was performed by means of a Pearson Correlation with the MBI-C and NPI-Q scales. Divergent validity was also studied using the same method with the GDS-15 Scale. The significance level was set at α =.05. A ROC curve was generated to determine the usefulness of the total test score for the diagnosis of MBI, as well as the sensitivity and specificity of the chosen cut-off point. The total test score was the contrast variable and the diagnosis of MBI the static variable.
Results
Internal Structure of the Test
The correlation matrix of the 38 initial items was analysed and the items that asked the same question in different ways or where the question was included in another simpler and more complete item and would therefore be redundant were eliminated (Ferrando et al., 2022; Lorenzo-Seva et al., 2021), until we were finally left with a total of 26 items. After this, successive Exploratory Factor Analyses (EFAs) were carried out, eliminating those items with factor loadings below .50. The quality indicators of the sample offer adequate results with a KMO (Kaiser-Meyer-Olkin) = .92 and a significant Bartlett's statistic (Chi-square 2277.6; P = .01). The final questionnaire would be composed of a single factor that includes the 19 final items of the test and explains 48% of the variance of the model. Finally, the fit of the statistical model to the data is correct, with a GFI = .97, a RMSEA = .068, a CFI = .98 and a NNFI = .98. The unidimensionality indicators give adequate values with UniCo = .97, ECV = .86 and MIREAL = .23. In Table 2 we can see the factor weights of each item. It should be noted that none of the test items presented Differential Functioning with respect to the sex variable.
Table 2. Factor Loadings (FL) of the Unifactor Model
| Item | CF |
|---|---|
| ¿La persona ha perdido el interés en los amigos o la familia? [Has the person lost interest in friends or family?] | .73 |
| ¿La persona ha dejado de hacer actividades que antes realizaba: jugar a las cartas, pasear, leer, ver la tele? [Has the person stopped doing activities that he/she used to do: playing cards, walking, reading, watching TV?] | .58 |
| ¿La persona está menos activa que antes? [Is the person less active than before?] | .72 |
| ¿La persona está más preocupada o nerviosa por cosas rutinarias: ir al médico, hacer la compra, ir de viaje, etc.? [Is the person more worried or nervous about routine things: going to the doctor, shopping, going on a trip, etc.?] | .60 |
| ¿A la persona le cuesta más relajarse o está más inquieta que antes? [Does the person find it more difficult to relax or is the person more restless than before?] | .70 |
| ¿La persona se siente más nerviosa o inquieta cuando no tiene a una persona cercana (familiar, amigo o cuidador) a su lado? [Does the person feel more nervous or restless when they do not have a close person (family member, friend or caregiver) by their side?] | .66 |
| ¿La persona está más triste o baja de ánimo que antes? [Is the person sadder or more low-spirited than before?] | .67 |
| ¿La persona disfruta menos de lo que hace que antes? [Does the person enjoy what he or she does less than before?] | .69 |
| ¿La persona ha empezado guardar, acumular cosas o comprar cosas innecesarias o en exceso? [Has the person started storing, hoarding or buying unnecessary or excessive things?] | .56 |
| ¿A la persona le cuesta abandonar lo que está haciendo una vez empieza a hacerlo? [Does the person find it hard to give up what they are doing once they start doing it?] | .61 |
| ¿Cree que la persona tiene una idea en su cabeza que repite constantemente? [Do you think the person has an idea in his or her head that he or she keeps repeating?] | .71 |
| ¿La persona se ha vuelto más desconfiada respecto de otras personas? [Has the person become more distrustful of other people?] | .69 |
| ¿La persona está más irritable que antes? [Is the person more irritable than before?] | .77 |
| ¿La persona se muestra muy impaciente constantemente o se enfada ante retrasos? [Is the person constantly very impatient or angry about delays?] | .72 |
| ¿La persona se ha vuelto más insensible respecto a los demás? [Has the person become more insensitive towards others?] | .76 |
| ¿Se comporta con personas desconocidas como si fueran amigos o familiares? [Does he or she behave towards strangers as if they were friends or family?] | .59 |
| ¿Ha observado si ahora es más caprichoso o egoísta, que solo piensa en él/ella mismo/a? [Have you noticed if he/she is now more capricious or selfish, thinking only of him/herself?] | .62 |
| ¿Le cuesta comprender a los demás y no muestra preocupación cuando tienen una desgracia o un problema? [Do you find it difficult to understand others and show no concern when they have a misfortune or a problem?] | .60 |
| ¿Ha notado si la persona descuida su higiene personal en los últimos meses? [Have you noticed if the person has neglected personal hygiene in recent months?] | .71 |
Test Reliability
The reliability of the test scores is high, with a Cronbach's Alpha = .94 and McDonald's Omega = .97.
Evidence of Validity of Relationship With Other Variables
When studying convergent validity with other tests, a significant correlation was found between the screening test and the MBI-C and NPI-Q scales, and a low correlation with respect to the GDS-15 scale, both completed by relatives and by the patient him/herself, being higher in the last one (see Table 3).
Table 3. Evidence of Convergent and Divergent Validity of the CO-DCoL Scale
| Scale | MBI-C | NPI-Q | GDS-15 Fa | GDS-15 Pb |
| CO-DCoL | .88 | .82 | .36 | .56 |
a GDS-15 F: GDS-15 completed by reporter
b GDS-15 P: GDS-15 completed by patient
Given that there is a certain correlation between our test and the GDS-15 scale, we decided to check whether the correlation between our test and the MBI-C and NPI-Q tests is being affected by the former. To this end, we performed partial correlations between the CO-DCoL, the MBI-C and the NPI-Q, controlling for the effect of the GDS-15 (see Table 4). However, despite the elimination of the effect of this test, we still found a high correlation between the MBI-C and the NPI-Q and the CO-DCoL.
Decision Validity Evidence: ROC Curves
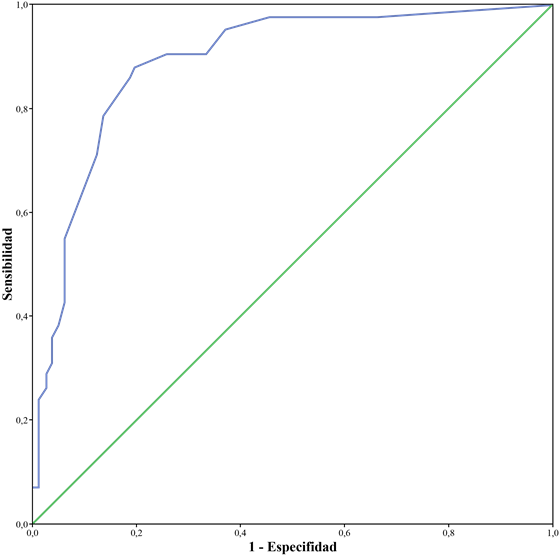
The ability of the test to detect DCoL was evaluated and a ROC curve with a good discriminatory ability was found, with an Area Under the Curve (AUC) of .89 (see Figure 1). A cut-off point of 6.5 was established to find a balance between sensitivity (.88) and specificity (.80), giving greater weight, however, to the sensitivity of the test.
Discussion
In clinical practice, it is common to find ourselves in doubt as to whether, when assessing behavioural symptoms in the elderly population, these are due to psychological factors such as anxiety (Bryant et al., 2008) or depression (Barua et al., 2011) caused, in many cases, by life situations such as the loss of family and friends, retirement, reduction of social relationships, etc., or whether we are dealing with a NPS as an early manifestation of dementia. Special mention should be made of the elderly population who repeatedly attend psychiatry consultations or present with late-onset psychiatric symptoms, as these are highly likely to be associated with incipient deterioration (Pink et al., 2015; Cieslak et al., 2018; Wise et al., 2019). Correct identification of NPS is crucial at this time for referral to the most appropriate speciality for initiation of treatment. Despite the existence of different standardised tests, the lack of experience in the application of these tests or the limited time available in consultations, among other factors, means that the most common criterion in the diagnosis of these NPS is the opinion of experts and their experience in the field (Cummings, 2021). In this sense, having adequate, quick and easy-to-use screening tools is a point in favour of the professional and the patient for the correct identification of symptoms. At present, there are different standardised tests that allow an adequate approach to this symptomatology, including the MBI-C (Aguera-Ortiz et al., 2017) and the NPI-Q (Boada et al., 2002). Regarding the use of the NPI-Q questionnaire for the diagnosis of NPS in preclinical and prodromal stages, it was initially developed for behavioural symptoms of dementia, and therefore does not follow the current ISTAART criteria for diagnosis in earlier stages. The MBI-C has been developed following these criteria, but its main weakness is the high number of items (34), which makes it difficult to use as a screening test.
Therefore, the aim of this study was to develop an instrument for the elderly population specifically for the assessment of MBI that could be used as a screening test in the first consultation with patients, thus helping to identify these NPS, as prodromes of a neurodegenerative pathology, and thus supporting cognitive screening tests to facilitate a more efficient early diagnosis.
The development of the questionnaire was based on the application of current knowledge about different NPS present in the preclinical and prodromal phases of dementias. Based on this, the initial 50 items were constructed which, as indicated above, were reduced to 38 after comments from different experts. Subsequently, an exploratory factor analysis allowed us to eliminate redundant items (Ferrando et al., 2022; Lorenzo-Seva et al., 2021), as well as those with little relation to the diagnosis of MBI. Dimension five of the ISTAART criteria (abnormal perceptions) was poorly represented in the final version of the test due to the low prevalence of symptoms in the study sample. However, this can be easily explained as the NPS associated with this dimension (psychotic-like symptoms) are usually present in advanced stages of neurodegenerative pathologies and are rarely present in preclinical or prodromal stages of the disease (it is an essential diagnostic symptom in Lewy Body Degeneration (Ballard et al., 2013)).
The final questionnaire consists of only 19 items and is quick and easy to complete. Although the ISTAART criteria have five different domains, the test shows a single factor in which all 19 items are grouped. Nevertheless, the different domains are represented to a greater or lesser extent in these items. The existence of a single factor may be due to two reasons: on the one hand, the small number of items may make it impossible to group them into 5 distinct factors; on the other hand, the domains of the ISTAART criteria are theoretical and non-statistical constructs that seek to group the NPS representative of the MBI pathology and, therefore, are not a "psychometric" construct as such. The questionnaire, in this sense, would not necessarily have five distinct factors.
It should be noted that the questionnaire has been developed to be completed by the patient's companion who should report the symptoms he or she perceives in the patient. This is done to avoid bias in the information provided to the professional in those patients with little awareness of the deficits (Chen et al., 2022) and which may lead to an incorrect interpretation of the results of the questionnaire by the professional. In this sense, it is worth noting a very good reliability of the test with an α = .94 and an Ω = .97. With regard to the convergent validity of the questionnaire, high correlations have been found with both the MBI-C and the NPI-Q. On the other hand, divergent validity has been assessed with respect to the questionnaire for the assessment of depressive symptoms in the elderly population GDS-15, finding a modest correlation both in the test completed by relatives and by the patient him/herself. This would allow us to rule out the possibility that we are dealing with a test that only measures depressive symptomatology and not the MBI as such.
In the case of convergent validity, the MBI-C and the CO-DCoL measure Mild Behavioural Impairment following the criteria of the ISTAART, with the formulation of some items being similar, although with greater simplicity (without double questions in the case of the MBI-C) and different response criteria. In the MBI-C the response would be based on the severity and speed of onset of the symptom, while in the case of the CO-DCoL it would be based on the severity and the disruption that the symptom generates in the patient's environment and in the patient's own environment. With respect to the NPI-Q, this correlation is explained by the fact that the NPS under study, although assessed on the basis of different criteria, are for the most part coincidental (both measure apathy, depression, etc.), with similar results having been found in other studies comparing the NPI-Q and the MBI-C (Mallo et al., 2019). The correlation of the NPI-Q with the CO-DCoL is lower than with the MBI-C, probably because the former does not follow the ISTAART criteria that require a minimum of 6 months of symptom maintenance, being only one month in the case of the NPI-Q and causing an overestimation of the prevalence (Sheikh et al., 2018) and a lower correlation with the test to be developed. With respect to the GDS-15 Scale, it is to be assumed that, although there is necessarily some correlation, given that the first and second domains of the MBI (decreased motivation and affective dysregulation) are symptoms clearly associated with depressive symptomatology (Fiske et al., 2009), this correlation is not significantly high and even once the effect of this type of symptomatology is discounted, there is still a high correlation with the MBI-C and the NPI-Q.
Finally, the CO- DCoL has shown in the present study a good sensitivity (.88) and specificity (.80) with a cut-off point of 6.5. This cut-off point seeks a balance between the sensitivity and specificity of the test, although giving greater relevance to sensitivity to avoid an underestimation of the MBI in the population and reducing the number of patients who are "left out of the diagnostic process" after the first consultation.
The present study would confirm the usefulness of the CO-DCoL as a possible screening test for Mild Behavioural Impairment that can be used as a complementary tool to cognitive screening tests for a better early identification of neurodegenerative diseases. This test would help the professional to further discriminate psychological symptoms in the older population from those that may be NPS in preclinical and prodromal stages of the disease. Furthermore, the identification of MBI by means of this questionnaire would reinforce the need to continue the study by the neurology area even in the absence of cognitive impairment found in cognitive screening tests, as it is a risk factor for progression to dementia or a possible initial symptom of the disease (Taragano et al., 2018).
Despite the results obtained in this study, it is an initial study with a small population sample. This may be generating biases in the prevalence of symptoms, which may be the case of the criteria related to abnormal perceptions or alterations in the content of thought (hallucinations and delusions) in MBI. In this sense, future studies should increase the population sample, both of cognitively healthy patients and those with MBI, in order to know more precisely the psychometric properties of the test and its performance in different population groups. A second limitation to be taken into account is that the period in which the population sample was collected coincided with the years of the COVID-19 pandemic, and during this time an increase in NPS due to social isolation was observed (Simonetti et al., 2020), which may be affecting the results of the study.