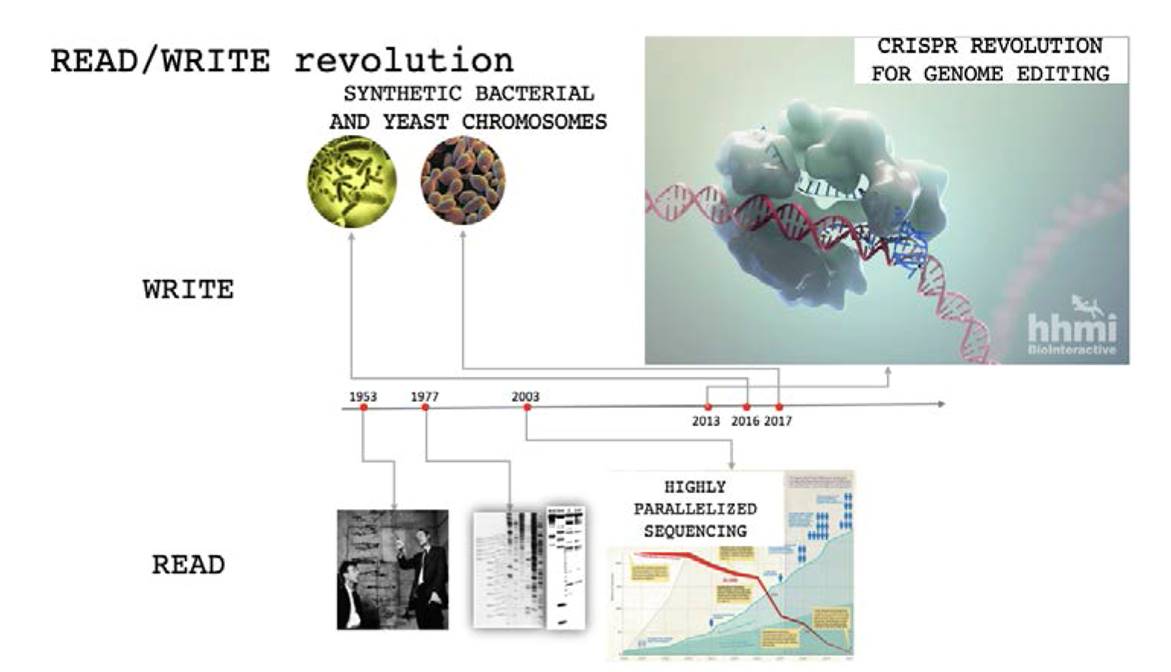
1. Read and write revolution
Over the last couple of decades, biotechnology has undergone a massive revolution. Biosciences have learned to interact with the biosphere with its own genuine language: DNA. It is what can be called the Read and Write revolution (Figure 1). Sequencing technologies have progressed from painful sequencing of short DNA messages to whole genomes which contain thousands of millions of bases. One human genome contains 3,000 times more characters than Don Quijote by Cervantes. Modern sequencing methodologies have massively parallelized the DNA reading process (Shendure et al. 2005). Nowadays, a human genome can be sequenced within a few hours and cost less than $1,000 ("DNA Sequencing Costs: Data | NHGRI" n.d.). Reading DNA allowed biosciences to better understand life.
In parallel, biosciences have learned how to rewrite DNA. DNA synthesis costs have dropped dramatically ("Bioeconomy Dashboard - Bioeconomy Capital" n.d.). Full de novo synthesis of bacterial genomes (Ostrov et al. 2016; Gibson et al. 2008) and eukaryotic chromosomes (Pretorius and Boeke 2018) have been performed. Also, biosciences have learned how to edit genomes. Thanks to tools like CRISPR (Jinek et al. 2012), we can modify specific parts of this massive book which is the human genome. CRISPR technology was first demonstrated for editing human genomes in vitro in 2013 (Mali et al. 2013; Cong et al. 2013), and deployed clinically in the US in 2019 (Darie 2019). This capacity of modifying the biosphere has advanced tremendously causing impacts in research, medicine, and industry. Genome editing technologies such as CRISPR has accelerated genome engineering in research. The number of scientific publications indexed in PubMed mentioning CRISPR has increased exponentially, being over 5,000 on the year 2018 (Adli 2018). The increased capacity of genome engineering has also translated into clinical use. In 2018, several thousands of gene therapy trials were being conducted in the world (Ginn et al. 2018). Rewriting DNA enables biosciences to modify life.
2. Therapeutic uses of gene editing
Therapy is probably one of the most impactful write enabling indications. This practice has a wide support from the scientific community (Baltimore et al. 2015). Novel methodologies to repair faulty human genomes causing genetic diseases or genetically enhancing humans to attack cancer have been developed. Human metagenome editing is also being exploited for therapeutic purposes by equipping our microbiota with therapeutic genetic modifications. Additionally, advanced genome writing in animals such as pigs is providing with a new platform of organs and tissues for transplantation.
2.1. Repairing faulty genomes
Editing genomes has the potential of transforming a sick genome into a healthy genome. First applications of gene therapy have targeted the curation of genetic diseases. The most common case of such diseases is the inheritance by an individual of two recessive mutant alleles from each progenitor. One example is Leber congenital amaurosis (LCA) which is one of the major causes of blindness among children. Mutations in the CEP290, CRB1, GUCY2D or RPE65 are the most common causes of this disorder (Tsang and Sharma 2018). In 2017, U.S. Food and Drug Administration (FDA) approved Luxturna (Spark Therapeutics) a gene transfer therapy where a healthy copy of RPE65 is delivered to the diseased retina using an Adeno-Associated Viral vector (Smalley 2017). This modified genome still has the two mutant RPE65 but it will have a third functional copy of RPE65. Editas Medicine is developing another medicine for variants of LCA caused by mutations on gene CEP290 which being 8kb cannot be packaged into an AAV vector. Editas team is trying to repair the genetic defect causing the disease by in situ editing using CRISPR/cas9 (Maeder et al. 2019). Clinical phase for this medicine will be the first in-body CRISPR medicine treatment (Sheridan 2018). Multiple aspects will have to be determined by the clinical phase including immunogenicity of cas9, impact of lifelong cas9 activity on the eye, or off target cas9 activity.
2.2. Synthetic genes in human genomes to fight disease
Not only faulty genomes can be repaired, but DNA write technologies can also enhance the human body to fight disease. A remarkable example is the chimeric antigen receptor T cell (CAR-T) therapies. T lymphocytes are genetically programmed to attack cancer by equipping them with a synthetic receptor containing a fusion of cancer recognition domain and multiple T cell activation signals (Castellarin et al. 2018). This technology has been clinically approved by the FDA and EMA for specific types of leukemia (Anonymous 2018) after highly successful clinical demonstrations (Maude et al. 2014). Novel versions of these CAR-T cells are extremely sophisticated with enhanced specificity and control encoded in advanced genetic circuits (Kitada et al. 2018).
CAR-T approach is expanded to other indications beyond such as HIV (Kuhlmann, Peterson, and Kiem 2018).
2.3. Editing human metagenomes
Humans are not pure eukaryotic individuals but ecosystems. Humans are ecosystems containing millions of microbes in multiple body sites such as our gut, skin or mouth. These microorganisms co-exist as commensals and perform essential metabolic and immune functions (Human Microbiome Project Consortium 2012). Each of these microbes has a genome. Write technologies can be used to edit host genomes but also of the microbes that live with the host. We can use microbes genomes to perform advanced synthetic functionalities. Early clinical demonstrations are being conducted by the company Synlogic. They have created a platform to engineer Escherichia coli to provide metabolic functionality to solve deficiencies of the host caused by a genetic disease. For instance, Synlogic create bacteria that can metabolize phenylalanine and help fight phenylketonuria (Isabella et al. 2018) or bacteria that eliminate ammonia to help fight urea cycle disorders (Kurtz et al. 2019). DNA write technologies enable to do gene therapy without modifying a single base of the human genome.
Deployment of genetically modified bacteria outside of the laboratory has caused environmental alarms. In order to control the propagation of these genetically modified bacteria, different biocontainment strategies are being used including auxotrophic markers (Mimee, Citorik, and Lu 2016).
2.4. Xenotransplantation
Only in the US, 20 people die every day waiting for an organ ("Transplant Trends - UNOS" n.d.). The lack of organs for xenotransplantation is one of the biggest unmet medical needs. Writing DNA may enable to produce an unlimited supply of organs in genetically modified pigs. Two problems hinder xenotransplantation: pig-to-human compatibility and presence of an endogenous virus in the genomes of pigs. The progress is astonishing. Pigs free of endogenous retroviruses have been produced (I) and genetically modified pigs organs last in non-human primate models up to years (II).
I. DNA write technologies have been used to eliminate porcines endogenous retroviruses or PERVs. Endogenous cannot be removed other than by genome engineering as they are transmitted vertically from parents to offspring. PERVs are an important concern for xenotransplantation applications as they can be transferred from pig-to-human (Güell et al. 2017). In 2015 we reported the elimination of 62 copies of PERVs in the pig genome (Yang et al. 2015), and in 2017 we went on to produce pigs with all PERVs inactivated (Niu et al. 2017).
II. Immune and physiologic engineering to increase pig-to-human compatibility is performed by genetic engineering as well. Important human genes are added to the pig genome and genes that pigs and humans do not have are removed. We already have pigs that produce hearts (Längin et al. 2018), kidneys (Iwase et al. 2017) and pancreatic islets (Aristizabal et al. 2017) that last for years in NHPs
Editing pig genomes for xenotransplantation could address probably the biggest unmet medical need, which is to provide an unlimited supply of organs for patients in need.
3. Beyond wide consensus: germline editing and genetic enhancement
During the Second International Summit on Human Genome Editing there was an unexpected communication. He Jiankui, a Chinese researcher stated that two girls, Lulu and Nana, had been born after they had been genetically modified. CRISPR cas9 was used, a relatively new technique to destroy the CCR5 receptor, the gateway to HIV (Xu et al. 2017). Somehow their resistance to HIV was genetically coded.
Yuval Harari, historian and visionary describe that Homo sapiens as we know it, will cease to exist. Artificial intelligence and biotechnology will bring the human species to another level. The author speaks of a Homo Deus that has become capable of modifying his or her own nature (Harari 2016). Humanity has used genetic engineering to modify human beings. Thousands of clinical trials are ongoing (Ginn et al. 2018), and several treatments have completed successful clinical phases and have been approved to cure different diseases (cancer, blindness, immunodeficiencies...). However, attempts have also been made beyond strictly therapeutic. Elisabet Parrish, CEO of Bioviva, a company that sells anti-aging treatments, decided to add to her genome extra copies of the telomerase gene (Regalado n.d.). This treatment has been shown to extend from 13 to 24% the lifespan of mice (de Jesus et al. 2012). Josiah Zayner, CEO of The Odin, injected himself CRISPR-based treatment to activate muscle growth (Ireland 2017). Although the nature of the treatments is based on solid scientific principles, no detailed results have been published. What has been different in Lulu and Nana? Why this case has generated so much more debate? These changes have occurred in the germline. Not only Lulu and Nana have been genetically modified, but also their offspring. The germline of human beings had never been modified beyond the early embryo stage.
Several aspects have been criticized for this experiment. First of all, the girls were to be born healthy. There is a large consensus in the scientific community that experimental therapies apply first to very serious diseases, situations where the potential benefits are much greater than the risks of a new therapy. In fact, the first uses of CRISPR cas9 are for sickle cell anemia (Banks 2018), blindness (Sheridan 2018), and cases of refractory cancers (Darie 2019). These girls have been exposed to totally unnecessary risk for a potential preventative benefit. We are still characterizing the risks associated with CRISPR therapies. Second, the effect of genetic changes is unknown. Nana seems to contain only one of the two modified homologous chromosomes. Therefore, it would not have achieved resistance to HIV. In addition, both in Nana and in Lulu, the specific edition introduced is not exactly the one found in nature that provides resistance to HIV. Third, the situation also exposed a self-regulatory error of the scientific community. During the I International Human Genome Edition Summit a moratorium on implanting modified embryos was established that He Jiankui has skipped. In spite of premeditation and the risk exposed, it is very likely that the girls are healthy. We are not in the face of the dramatic cases of the errors of the gene therapy of two decades ago where several people died ("Gene-Therapy Trials Must Proceed with Caution" 2016; Check 2002). However, it is a time of great reflection.
Will we have people modified by CRISPR in the germline? Highly probably, yes. But at the right time. We need sufficient scientific information (we do not yet have) and transparency. A global vision is needed to which the scientific community adheres. An informed debate is needed that includes the scientific community, regulatory bodies, and society to decide where we want to go as a society.
Where can we get? George Church, a geneticist from Harvard University, has compiled a list of genetic traits that may have an important impact on humans (http://arep.med.harvard.edu/gmc/protect.html). The list includes resistance to various infectious diseases, reduced aging, lower probability of cancer, etc. In fact, this list includes the genetic edits of Lulu and Nana (as well as those attempted by Elisabeth Parrish or Josiah Zayner). However, it is not likely that many humans will be modified with these changes over the next few years. This Homo Deus will still take some years to settle down. Much more likely we will see the application of CRISPR to cure serious illnesses in somatic cells.
4. Conclusions
Reading and writing DNA empowers us to change our world, even to change ourselves. Biotechnology is an essential part of the fourth industrial revolution (Schwab 2017), the potential benefits are enormous. A novel generation of therapeutics is emerging. Not only wan we repair genomes but also engineer humans to be more efficient in eliminating cancer or other diseases. Implications go beyond human therapy. Biology enables the re-design industrial processes to be more sustainable for our planet (French 2019). A switch to bioproduction with enhanced sustainability; or a new generation of food and luxury products, where meat and leather are grown on a dish rather than obtained after killing an animal.
This is an extraordinary opportunity for humanity. We need to accelerate debate including the stakeholders: society, scientific community, industry, and regulatory agencies to foster a responsible use of these technologies and maximize positive impact on society.