Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista de Osteoporosis y Metabolismo Mineral
versión On-line ISSN 2173-2345versión impresa ISSN 1889-836X
Rev Osteoporos Metab Miner vol.5 no.2 Madrid abr./jun. 2013
https://dx.doi.org/10.4321/S1889-836X2013000200004
Divergent effects of TGF-β inhibition in bone metastases in breast and lung cancer
Efectos divergentes de la inhibición de TGF-β en metástasis óseas de cáncer de mama y pulmón
Luis-Ravelo D., Antón I., Vicent S., Zandueta C., Martínez S., Valencia K., Ormazábal C., Lecanda F.
Laboratorio de Adhesión y Metástasis - División de Oncología - Centro de Investigación Médica Aplicada (CIMA)-Universidad de Navarra-Pamplona
Work scholarship from the SEIOMM to attend the 33 Congress of the ASBMR (Toronto, Canada. 2010).
This work was financed by the "FIMA UTE project" agreement, RTICC RD06/0020/0066, PI042282, FIT-090100-2005-46, SAF-2009-11280 and the "Ortiz de Landáruzi" Award (67/2055, Government of Navarra) and "Fundación La Caixa" obtained by F.L.D. L-R by FIMA and the FPU National Programme and I.A by FIMA and the Government of País Vasco. F.L is a researcher in the Programme 13.
SUMMARY
Background: The objective of this study lies in the determination of the validity of transforming growth factor β (TGF-β) as a therapeutic target in models of metastasis deriving from different histological types of lung cancer.
Material and methods: 4-week-old immunodeficient mice inoculated with lung and breast cancer lines were treated with cytokine inhibitor peptide, control peptide or placebo. Weekly bioluminescence and microradiographic measurements were taken to determine the effects of the treatment on tumor burden and metastatic lesions in the long bones.
Results: Treatment with the specific peptide against TGF-β has a protector effect in the bone of animals inoculated with the breast cancer lines, unlike what happens in the control peptide and placebo groups. However, the anti-TGF-β treatment lacks the significant therapeutic effects on the bone metastases which develop in lung cancer bearing animals.
Conclusions: The role of TGF-β as a potential therapeutic target in bone metastasis is highly dependent on the histopathological type and subtype of tumor.
Key words: TGF-β, bone, metastasis, animal models.
RESUMEN
Fundamento: El objetivo de este estudio radica en la determinación de la validez del factor de crecimiento transformante β (TGF-β) como diana terapéutica en modelos de metástasis óseas derivadas de distintos tipos histopatológicos del cáncer de pulmón.
Material y métodos: Ratones inmunodeprimidos de 4 semanas de edad inoculados con líneas de cáncer de pulmón y de mama fueron tratados con péptido inhibidor de la citoquina, péptido control o placebo. Se tomaron semanalmente medidas de bioluminiscencia y microrradiografías para determinar el efecto del tratamiento sobre la carga tumoral presente en los huesos largos y las lesiones metastásicas en los mismos.
Resultados: El tratamiento con el péptido específico frente a TGF-β tiene un efecto protector en el hueso en los animales inoculados con la línea de cáncer de mama, a diferencia de lo ocurrido en los grupos de péptido control y placebo. Sin embargo, el tratamiento anti-TGF-β carece de efectos terapéuticos significativos sobre las metástasis óseas que se desarrollan en los animales inoculados con las líneas de cáncer de pulmón empleadas.
Conclusiones: El carácter de TGF-β como posible diana terapéutica en metástasis en hueso es altamente dependiente del tipo y subtipos histopatológicos de tumor.
Palabras clave: TGF-β, hueso, metástasis, modelos animales.
Introduction
The skeleton is one of the preferred targets for tumour cells. Neoplasia of the breast, prostate, lung and myeloma very frequently generate bone metastases [1]. The prognosis for survival from a diagnosis of bone metastasis varies depending on the type of tumour. In patients with lung cancer with this type of metastasis, the average survival is measured frequently in months, being the lowest of all types of tumour with bone tropism [2]. Furthermore, on many occasions this phenomenon is detected at the time of diagnosis of the disease, a fact which contributes to this neoplasia being the primary cause of death from cancer [3].
The high frequency of bone metastasis may be explained by the endothelial fenestrations of the bone marrow, which could facilitate the establishment of the metastatic cells [4]. The bone is also a propitious environment for the development of these cells, since it is a medium rich in growth factors such as transforming growth factor β (TGF-β) [5]. The intracellular signalling route for this cytokine involves the phosphorylation of the Smad proteins, which ultimately allows the expression of the target genes in the nucleus. TGF-β has opposing functions in carcinogenesis. On the one hand, signalling through its receptor triggers an anti-proliferative response in conditions of oncogenic stress. This takes place through the induction of the expression of tumour suppressor genes such as the cyklin-dependent kynase inhibitors (CDKI) or the repression of oncogenes such as c-Myc and members of the ID family [6]. On the other, there are neoplasms which keep this pathway intact, evading this cytostatic response and simultaneously favouring tumour progression. Notable among other mechanisms is its contribution to the evasion of immunity mediated by the T CD8+lymphocytes [7] and angiogenesis by the induction of vascular endothelial growth factor (VEGF) and MMPs (matrix metaloproteases) in the tumour and the endothelium [8]. The tumour cell may also use TGF-β signalling for the progression of the metastasis in the bone microenvironment, given that this cytokine favours the expression of the protein related to the parathyroid hormone (PTHrP) or IL11 [9,10]. These factors induce the expression of RANKL (ligand for receptor activator for nuclear factor κκB) in the membrane of the osteoblasts. The recognition of the ligand for the corresponding receptor in the mononuclear precursors entails their activation and the formation of the mature osteoclasts. The action of these cells promotes the release from the bone matrix of cytokines which prime the metastatic colonisation. Thus a positive feedback process or "vicious circle" is generated which magnifies the osteolytic effects.
The genetic or pharmacological inhibition of the signalling pathway of TGF-β has been shown to have therapeutic benefits in preclinical models. The expression of the dominant negative form of the TGFBR2 receptor in breast tumour cells or the use of the TGFRI SD-208 inhibitor in animals inoculated with melanoma cells with bone tropism has shown a reduction in metastatic lesions in the bone which these cell lines occasion [11,12]. Similarly, our group demonstrated this effect in a model of bone metastasis derived from a large cell pulmonary carcinoma line [13].
The main objective of this study is to assess the contribution of TGF-β to bone metastasis in models derived from other histopathological models of lung cancer such as adenocarcinoma or carcinoid. A model of bone metastasis derived from breast cancer was used as a control.
Material and methods
Cell culture
Tumour cell lines A549, H727 and MDA-MB-231 from lung adenocarcinoma, lung carcinoid and breast cancer, respectively, were used. The lines A549 and H727 were transfected with the retroviral vector SFG-NES-TGL (kindly donated by Dr Ponomarev), which contains the luciferase reporter gene. The cells were cultivated at 37oC and 5% CO2 in sterile conditions in RPMI (A549 and H727) or DMEM (MDA-MB-231) medium, supplemented with 10% FBS, 100 units/ml of penicillin and 100 µg/ml of streptomycin (Invitrogen®).
Animals and intracardiac inoculation (I.C)
An injection of tumour cells was made into the left ventricle of 24 immunosuppressed mice, 4 weeks of age (Harlan Laboratories) in accordance with previous descriptions [14,15]. The cells were resuspended in PBS at a concentration of 2x106 cells/ml. The animals were anaesthetised intraperitoneally prior to the inoculation with ketamine (65 mg/kg) and xylacine (2.5 mg/kg). 2x105 cells (100 µl) were injected using a 29G calibrated needle.
All the protocols for working with laboratory animals were approved by the Ethics Committee for Animal Experimentation of the University of Navarra (CEEA).
Therapeutic regimen
Control peptides p41, or anti-TGF-β p17 or p144 (kindly donated by Digna Biotech as available) were used to assess this cytokine as a therapeutic target. Both had similar activity in vivo [13,16]. Five days after the inoculation of the tumour cell lines the animals were divided in 3 groups of 8 mice, each of which was to receive a daily dose, intraperitoneally, of 3.75 mg/kg of p144, control peptide (p41) or vehicle (physiological serum). The mice inoculated with the MDA-MB-231 line were divided equally, and from day 7 treated on alternate days with 2.5 mg/kg of p17 (similar activity to p144), control peptide p41 or vehicle.
The dose of p17 was chosen on the basis of its activity in previous studies [13,17], while that of p144 was higher to ensure that the results obtained in vivo with the lung cancer cell lines were not attributable to a low concentration of the peptide. The time period between the intracardiac inoculation and the start of the treatment had been established in earlier experiments, in which the time necessary for the cells to be detected in bone was determined, through bioluminescence or isolation of the metastatic cells.
The effect on the tumour was determined on a weekly basis through bioluminescence and/or osteolysis through X-ray analysis, looking for possible differences.
The duration of each experiment depended on the development of metastasis for each cell line.
Bioluminescence
D-luciferin (PROMEGA) was administered intraperitoneally at a concentration of 150 mg/kg to the previously-anaesthetised animals. After 5 minutes different images of bioluminescence were taken in a CCD chamber using the Living Image (IVIS® system, Xenogen) programme. The same programme was used to quantify the bioluminescent signal defining the lower extremities. From the data obtained was subtracted the luminometric value of a mouse not injected with cells (control). The values obtained were divided by the luminometric value of each extremity obtained before the start of treatment. The luminometric signal obtained appears superimposed on the rodent.
The cells transfected with SFG-NES-TGL, A549 y H727 vectors had a detectable signal. No tumour-related luminometric data from the animals inoculated with the MDA-MB-231 line was obtained, given that the vector with the luciferase gene with which it was transfected did not generate a signal, probably due to the methylation of the promoter [13].
Radiographic analysis
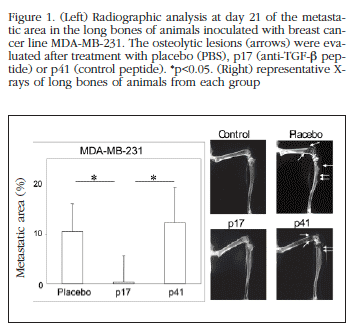
The X-rays were carried out under anaesthetic using a Faxitron® (MX-20) X-ray model. Film sensitive to this radiation (MIN-R, Kodak®) was used at 20kV for 20 seconds at 2x magnification. The radiographs were digitized at a resolution of 1200 ppi (Epson® Expression 1680 Pro). The area of osteolytic lesions was analysed with the computerised image analysis programme AnalySIS® (GmbH). The relative quantification of the metastatic areas was expressed as the percentage of the sum of the areas of lesion in the femur and tibia with respect to the total surface area of these long bones on the films.
Computerised microtomography (µCT)
Femoral and tibial joints representative of each experimental group were analysed in a microCT device (micro CAT II, Siemens® Preclinical Solutions) at 75.0 kVp and 250 uA. Each scan was carried out at a resolution of 10 µm. The two-dimensional images were reconstructed using a standard deconvolution procedure with a Shepp-Logan filter. For the reconstruction of the images the COBRA®_Exxim progamme was used. The images were stored in three-dimensional frames with a voxel size of 19*19*23 µm.
Statistical analysis
The SPSS 15.0 software programme was used to determine the statistical value of the results. A significance level of α=0.05 was used. Values of p lower than this limit were considered as significant (*). A single factor ANOVA analysis was carried out to study the metastatic area of the radiographs with multiple Tukey comparisons and the Kruskal-Wallace test followed by multiple comparisons with Mann-Whitney for bioluminescence. The p values obtained were adjusted with the Bonferroni method.
Results
The radiological analysis of the femurs and tibias of the animals inoculated with the MDA-MB-231 line showed a drastic reduction in the metastases of the animals treated with p17 in comparison with those treated with control peptide or placebo three weeks from the initiation of the experiment (Figure 1). In the animals inoculated with the H727 line, the bioluminescence images at day 22 showed a slight effect of p144 on the tumour load in the lower extremities of the animals treated with this peptide (*p< 0.05, Figure 2A). This effect lost its significance in the days following the experiment (Figure 2B). Similarly, the radiographical analysis of the long bones revealed a slight reduction in metastatic lesions in these mice which was not significant (Figures 3A and 3B). On the other hand, in the animals inoculated with the A549 line the p144 did not demonstrate any protective effect on the tumour load determined by bioluminescence (Figures 4A and 4B) or on the development of osteolytic lesions (Figures 5A and 5B). These results indicate that the prometastatic activity of TGF-β is highly dependent on each cell line.
Discussion
Metastasis involves the acquisition of new functions on the part of the cell in the primary tumour. These include motility and invasion, intravasation, survival in circulation, adhesion to the endothelium, extravasation and growth or colonisation of the target organs [18]. This requires a genetic and/or epigenetic programme -little understood at present- influenced by the selective pressure established in the tumour itself and its microenvironment. Therefore, the identification of the targets involved in metastasis is critical. Knowledge of the factors effecting this process could allow the development of new antimetastatic therapies which would have an impact on the quality of life of cancer patients. This fact justifies the development of models of metastasis which reproduce clinical reality. The model based on intracardiac inoculation recapitulates the final stages of metastasis: extravasation, homing, and colonisation of target organs. With this technique a high and rapid incidence of metastasis with reproducible results is achieved. This approximation has been used before with tumour lines of melanoma, prostate and breast [10,19]. The main limitation is the exclusion of the initial events of the metastatic cascade such as invasion, motility and intravasation towards the pulmonary parenchyma. The models which recapitulate these initial stages of metastasis such as orthopaedic injection have been successful for breast cancer. Here, an extirpation of the primary tumour after its growth is carried out to facilitate the later appearance of metastases at a distance [20]. However, this approximation is not viable in the case of lung cancer, since the death of the animals frequently ensues before the development of the metastasis, due to the rapid growth of the tumour. Furthermore, the frequency of bone metastasis in orthotopic models is low.
In spite of the importance of TGF-β in the bone microenvironment and in metastasis, its therapeutic potential should be treated with caution. Firstly, the systemic inhibition of this pathway may generate collateral affects, since signalling through this pathway is of vital importance in the homeostatic process of tissues. Secondly, the results obtained show that the effects of this pathway depend on the cellular context. Breast tumour cells evade TGF-β's inhibitory signals, keeping intact the route to the limbs which favours the prometastatic function of the cytokines. The great abundance of transcriptional inhibitors of the genes involved in the cytostatic response in these cells may account for this phenomenon [21]. This may explain the therapeutic improvement obtained in the model of bone metastasis of the MDA-MB-213 cell line. As occurs with breast cancer, pulmonary oncogenesis may involve the loss of the tumour-suppressant effects TGF-β [22]. Therefore, this cytokine may be of therapeutic interest for the treatment of bone metastasis of lung cancer. In agreement with these results, the use of a peptide inhibitor of TGF-β in a model of large cell carcinoma showed a reduction in bone metastasis [13]. However, the results shown in this work demonstrate that this effect on adenocarcinoma and carcinoid of the lung are of little or no significance. These experiments substantiate therefore the cell -dependent context of the TGF-β pathway. Furthermore, it is possible that other cytokines present in abundance in the bone, such as IGF-1 [23], may constitute key elements for the progression of the vicious circle and consequent metastatic colonisation of bone. Future studies may determine other ideal targets for the development of antimetastatic therapies.
![]() Correspondence:
Correspondence:
Fernando Lecanda
Centro de Investigación Médica Aplicada (CIMA)
Universidad de Navarra
Avda. Pío XII, 55
31008 Pamplona (España)
Correo electrónico:
flecanda@unav.es
Date of receipt: 27/02/2013
Date of acceptance: 29/04/2013
Bibliography
1. Vicent S, Luis-Ravelo D, Antón I, Hernández I, Martínez S, de las Rivas J, et al. Bone metastases. An Sist Sanit Navar 2006;29:177-88. [ Links ]
2. Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res 2006;12(20 Pt 2):6243s-9s [ Links ]
3. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [ Links ]
4. Nguyen DX, Bos PD, Massagué J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer 2009;9:274-84. [ Links ]
5. Baylink DJ, Finkelman RD, Mohan S. Growth factors to stimulate bone formation. J Bone Miner Res 1993;8 Suppl 2:S565-72. [ Links ]
6. Padua D, Massagué J. Roles of TGFbeta in metastasis. Cell Res 2009;19:89-102. [ Links ]
7. Thomas DA, Massagué J. TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell 2005;8:369-80. [ Links ]
8. Sánchez-Elsner T, Botella LM, Velasco B, Corbí A, Attisano L, Bernabéu C. Synergistic cooperation between hypoxia and transforming growth factor-beta pathways on human vascular endothelial growth factor gene expression. J Biol Chem 2001;276:38527-35. [ Links ]
9. Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer 2002;2:584-93. [ Links ]
10. Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordón-Cardo C, et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 2003;3:537-49. [ Links ]
11. Yin JJ, Selander K, Chirgwin JM, Dallas M, Grubbs BG, Wieser R, et al. TGF-beta signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J Clin Invest 1999;103:197-206. [ Links ]
12. Mohammad KS, Javelaud D, Fournier PG, Niewolna M, McKenna CR, Peng XH, et al. TGF-beta-RI kinase inhibitor SD-208 reduces the development and progression of melanoma bone metastases. Cancer Res 2011;71:175-84. [ Links ]
13. Vicent S, Luis-Ravelo D, Antón I, García-Tuñón I, Borrás-Cuesta F, Dotor J, et al. A novel lung cancer signature mediates metastatic bone colonization by a dual mechanism. Cancer Res 2008;68:2275-85. [ Links ]
14. Catena R, Luis-Ravelo D, Antón I, Zandueta C, Salazar-Colocho P, Larzábal L, et al. PDGFR signaling blockade in marrow stroma impairs lung cancer bone metastasis. Cancer Res 2011;71:164-74. [ Links ]
15. Luis-Ravelo D, Antón I, Vicent S, Hernández I, Valencia K, Zandueta C, et al. Tumor-stromal interactions of the bone microenvironment: in vitro findings and potential in vivo relevance in metastatic lung cancer models. Clin Exp Metastasis 2011;28:779-91. [ Links ]
16. Dotor J, López-Vázquez AB, Lasarte JJ Sarobe P, García-Granero M, Riezu-Boj JI, et al. Identification of peptide inhibitors of transforming growth factor beta 1 using a phage-displayed peptide library. Cytokine 2007;39:106-15. [ Links ]
17. Ezquerro I, Lasarte JJ, Dotor J, Castilla-Cortázar I, Bustos M, Penuelas I, et al. A synthetic peptide from transforming growth factor beta type III receptor inhibits liver fibrogenesis in rats with carbon tetrachloride liver injury. Cytokine 2003;22:12-20. [ Links ]
18. Bogenrieder T, Herlyn M. Axis of evil: molecular mechanisms of cancer metastasis. Oncogene 2003;22:6524-36. [ Links ]
19. Wu TT, Sikes RA, Cui Q, Thalmann GN, Kao C, Murphy CF, et al. Establishing human prostate cancer cell xenografts in bone: induction of osteoblastic reaction by prostate-specific antigen-producing tumors in athymic and SCID/bg mice using LNCaP and lineage-derived metastatic sublines. Int J Cancer 1998;77:887-94. [ Links ]
20. Hoffman RM. Orthotopic metastatic mouse models for anticancer drug discovery and evaluation: a bridge to the clinic. Invest New Drugs 1999;17:343-59. [ Links ]
21. Gomis RR, Alarcón C, Nadal C, Van Poznak C, Massagué J. C/EBPbeta at the core of the TGFbeta cytostatic response and its evasion in metastatic breast cancer cells. Cancer Cell 2006;10:203-14. [ Links ]
22. Jeon HS, Jen J. TGF-beta signaling and the role of inhibitory Smads in non-small cell lung cancer. J Thorac Oncol 2010;5:417-9. [ Links ]
23. Yoneda T, Williams PJ, Hiraga T, Niewolna M, Nishimura R. A bone-seeking clone exhibits different biological properties from the MDA-MB-231 parental human breast cancer cells and a brain-seeking clone in vivo and in vitro. J Bone Miner Res 2001;16:1486-95. [ Links ]











 texto en
texto en