Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista de Osteoporosis y Metabolismo Mineral
versão On-line ISSN 2173-2345versão impressa ISSN 1889-836X
Rev Osteoporos Metab Miner vol.6 no.2 Madrid Abr./Jun. 2014
https://dx.doi.org/10.4321/S1889-836X2014000200005
REVIEW
Endocrine regulation of energy metabolism by bone
Regulación endocrina del metabolismo energético a través del hueso
González-Rozas M.1 and Pérez Castrillón J.L.2
1 Servicio de Medicina Interna - Hospital General de Segovia
2 Servicio de Medicina Interna - Hospital Universitario Río Hortega (Valladolid) - Departamento de Medicina de la Universidad de Valladolid
SUMMARY
The classical functions of bone are the maintenance of phosphorus-calcium homeostasis, damage repair, as well its structural function which allows locomotion and protects the vital organs. The recent discovery of new functions for bone in the regulation of energy metabolism suggest that bone may be an endocrine organ.
In the last decade, different genetic and molecular studies carried out in mice have determined that osteocalcin increases the secretion of insulin, and sensitivity to it, by increasing the secretion of adiponectin, stimulates the proliferation and the better functioning of the beta cells, promotes the reduction of fatty mass and an increase in the consumption of energy.
These findings demonstrate the existence of a reciprocal regulation between bone and energy metabolism, mediated by osteocalcin. The recognition of the metabolic role of osteocalcin is a significant discovery in the field of osteology and endocrinology, bringing the possibility of new therapies in the treatment and prevention of metabolic diseases such as diabetes mellitus, sarcopenia, obesity and osteoporosis.
Key words: osteocalcin, bone, endocrine organ, energy metabolism, insulin, diabetes mellitus, obesity.
RESUMEN
Las funciones clásicas del hueso son el mantenimiento de la homeostasis fosfo-cálcica, la reparación de los daños así como un efecto estructural que permite la locomoción y protege los órganos vitales. Los recientes descubrimientos de las nuevas funciones del hueso en la regulación del metabolismo energético sugieren que el hueso puede ser un órgano endocrino.
En la última década, en diferentes estudios genéticos y moleculares realizados en ratones, han determinado que la osteocalcina aumenta la secreción de insulina y la sensibilidad a ésta, a través de la elevación de la secreción de adiponectina, estimula la proliferación y el mejor funcionamiento de las células beta, promueve la reducción de masa grasa y el incremento del consumo de energía.
Estos hallazgos demuestran la existencia de una regulación recíproca entre el hueso y el metabolismo energético, mediada por la osteocalcina. El reconocimiento del papel metabólico de la osteocalcina, supone un descubrimiento importante en el campo de la Osteología y la Endocrinología posibilitando nuevas terapias en la prevención y tratamiento de enfermedades metabólicas como la diabetes mellitus, la sarcopenia, la obesidad y la osteoporosis.
Palabras clave: osteocalcina, hueso, órgano endocrino, metabolismo energético, insulina, diabetes mellitus, obesidad.
Introduction
The classical functions of bone are the maintenance of phosphorus calcium homeostasis, the repair of damage to the bone, as well as a structural function which allows locomotion and protects vital organs [1]. The bone is a dynamic tissue in constant change through bone remodelling, and which requires a great quantity of energy to perform this process [1-3].
Osteoporosis is the most frequent disease caused by changes in bone remodelling and is related to an increase in bone resorption or a decrease in its formation. The clinical observation that osteoporosis happens as a consequence of gonad failure and that being overweight protects against the development of osteoporosis suggests a hypothesis that appetite or body mass, reproduction and bone mass may have a hormonal regulation mechanism in common. This conjecture, and the recent discoveries of the new functions of bone in energy metabolism, suggest that bone may be an endocrine organ.
In the last few decades, numerous clinical trials have demonstrated that leptin, a hormone deriving from adipocytes, regulates the appetite and exerts a bimodal antagonistic effect on bone remodelling. To achieve this, two different hypothalamic pathways are used, the sympathetic nervous system (SNS) and the cocaine and amphetamine regulated transcript system (CART), which act directly on the osteoblasts [2].
Lee et al., in different genetic and molecular studies carried out in mice, determined that osteocalcin increases the secretion of insulin and its sensitivity by raising the secretion of adiponectin; and that it also stimulates the proliferation and functioning of the beta cells, at the same time as promoting the reduction of fat mass and an increase in the consumption of energy [4,5]. These findings demonstrate the existence of a regulatory relationship between bone and energy metabolism, mediated by osteocalcin (OC).
In some previous studies carried out in humans, different markers for low bone mass were detected in diabetic patients, among which was OC, but until very recently, research had not been carried out in these patients to determine its metabolic functions. Recently, an association has been found in adults between concentrations of OC and markers for metabolic syndrome and obesity, confirming the existence of an inverse relationship between OC and fat mass and levels of glucose [6].
This reciprocal relationship between bone and energy metabolism, with the recent recognition of the metabolic role of OC, is a significant discovery in the field of osteology and endocrinology, making possible new therapies for the prevention and treatment of metabolic diseases such as diabetes mellitus, sarcopenia, obesity and osteoporosis.
Bone is an endocrine organ
An endocrine organ may be defined as one capable of regulating development, integrating the functions of diverse organs and tissues, and coordinating the metabolic process of an organism by means of the synthesis and release of hormones secreted into the circulation. These regulatory functions are performed through feedback mechanisms in which the concentrations of the hormone itself indicate the necessity of increasing or reducing its production. This function is a fundamental characteristic of endocrine organs.
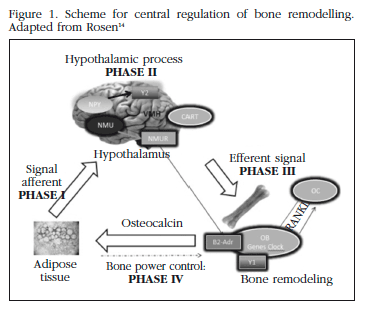
Bone remodelling is a biphasic process which occurs sequentially and in a balanced way. It consists of destruction or resorption followed by formation of new bone matrix [7,8]. This process allows the constant maintenance of bone mass throughout adulthood, and is essential for the maintenance of bone architecture to meet mechanical requirements, the repair of damage which occurs in daily exercise and the maintenance of the homeostasis of the phosphorous-calcium metabolism, such that remodelling constitutes a true homeostatic function [7-9]. A great many homeostatic functions, such as appetite and reproduction, are controlled by the hypothalamus. It is not strange to imagine that remodelling could, at least in part, be controlled by a central mechanism [9].
Bone remodelling requires a large and continuous supply of energy to the bone cells [1]. To cover this huge energy cost there needs to be co-regulation between energy metabolism and bone. In other words, bone remodelling may play a significant role in how glucose and energy are managed in the body [7].
These two biological aspects (the establishment of bone remodelling as a homeostatic function and its central control), along with their participation in the regulation of energy and glucose, suggest the hypothesis that there is regulatory coordination between bone remodelling and energy metabolism, probably through a central mechanism [8,9].
In the identification of hormones which may regulate the formation of bone, we start with two essential clinical facts. First, osteoporosis originates with gonad failure [10], and second, being overweight appears to protect against osteoporosis [11-13]. These two observations suggest the existence of a common regulatory mechanism between appetite, reproduction and bone mass. In attempting to determine the regulatory hormone or hormones, it has been observed that leptin is the only one which significantly influences all three functions.
It has been established that there are different phases in the central regulation of bone remodelling. The process starts with the emission of afferent signals from the adipocytes to the hypothalamus, in which leptin has an important role. It continues with a complex hypothalamic neural phase in which numerous neuropeptides participate, and from which originate efferent signals towards the adrenergic β2 receptors in the osteoblasts. As a consequence there are changes in transcription factors and clock genes which affect osteoblastogenesis. The last phase is the regulation by bone of the adipocytes, probably through the release of OC. The adipocytes may, in turn, regulate the proliferation and differentiation of the osteoblasts [14] (Figure 1).
Osteocalcin (OC)
Bone and energy metabolism appear to have a reguation which is linked by a central component, which is fat. To validate this hypothesis, a mediator is necessary which, originating in bone, is able to regulate energy metabolism. This mediator is OC.
The strategy for demonstrating this theory requires the identification of genes specific to the osteoblasts, to produce mice with deletions and to study the metabolic phenotypes [15]. Lee et al., in different experiments in vitro, confirmed that osteoblasts secrete a substance which affects the pancreatic cells and the adipocytes, and which appears to regulate glucose metabolism4. The co-cultivation of osteoblasts of wild-type mice with pancreatic islets increased by up to 500% the expression of the gene for insulin and of the markers for cell cycle progression in the islets. Karsenty et al., were the first to propose, in 2006, that there was endocrine regulation of energy metabolism in the skeleton [1].
The observation that a mouse deficient in protein specific to the osteoblast cell line, OC, (OC-/-), showed an abnormal quantity of visceral fat, led to the hypothesis that this was the hormone secreted by the osteoblasts which affected glucose metabolism [4].
OC is one of the few proteins specific to the osteoblasts which has numerous characteristics of a hormone. It is a molecule of low molecular weight (5,700 Da) produced by the osteoblasts4. It is present in all vertebrates, and is considered to be a marker for differentiation to mature osteoblasts. It is secreted into the circulation and, since its identification 30 years ago, has been considered to be the main constituent of the extracellular matrix, where it bonds with hydroxyapatite by means of three gamma-carboxylate residues, called Gla residues. This carboxylation offers an opportunity for regulation [16]. Surprisingly, although it is the most abundant non-collagen protein (15% in bone), it is not involved in bone formation [17].
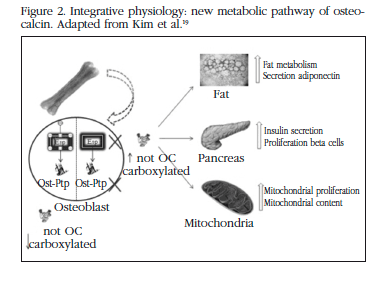
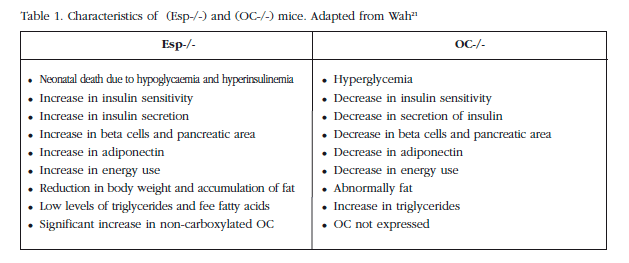
Mice without OC have high levels of glucose, low levels of insulin and a reduction in insulin secretion stimulated by glucose, in comparison with those with the wild genotype [4,20,21] (Figure 2). In the pancreas, the size of the islets, the mass of the beta cells and the amount of insulin were reduced. In addition, fat mass, the number of adipocytes and levels of triglyceride were increased. The expression of adiponectin and its molecular targets were reduced, and a subcutaneous infusion of recombinant OC in wild mice would produce an increase in insulin and in its sensitivity, and improve the expression of the insulin genes [4].
Subsequently, mice were obtained which had an absence of genes which are expressed preferentially in the osteoblasts. The first gene was Esp, which codes for the receptor of the tyrosine phosphatase protein (OST-PTP) present in mother cells, Sertoli cells and in osteoblast, and which are not expressed in the beta cells of the pancreas or in adipose tissue [2,4,18]. Those mice without Esp (Esp-/-) had larger and more numerous pancreatic cells than wild mice, proliferating by 60% to 300% in mice from the age of 5 days to one month. The increase in levels of insulin and insulin sensitivity was due to the capture of glucose in the muscle, brown fat, white fat and liver, and to the increase in adiponectin (the only adipokine affected, which was doubled in these mice), as well as an increase in the expression of other target genes of adiponectin, which increased threefold, such as the acyl-CoA oxydase gene, the gene for the receptor activator of the proliferation of alpha peroxisome (PPAR alpha), and the gene for the uncoupling protein 2 (UCP 2). On the other hand, those genes which coded for insulin-sensitising enzymes, as well as for adiponectin and insulin, were reduced in those mice without OC [19]. In mice without Esp an up to threefold increase was observed in the mitochondrial area and in proteins associated with biogenesis. In spite of insulin being a lipogenic hormone these mice were thinner due to an increase in energy use, evidenced by an increase in the expression of PGC1-alpha and of the uncoupling protein 1 (UCP 1), without the appetite being affected. The reduction in fat mass was restricted to visceral fat, and triglyceride levels were lower.
In order to confirm that the deletion of Esp would protect against the development of diabetic symptoms studies were carried out in mice in which hyperphagy was induced through hypothalamic structural lesions, destruction of beta pancreatic cells or feeding with a fatty diet. In the mice without Esp, it was confirmed that there was a lower weight gain and that no glucose intolerance or insulin resistance were developed [20].
The fact that the mice without OC have a phenotype contrary to those without Esp suggests that the two genes are in the same pathway, although they do not physically interact (Table 1). Lee reported that 90% of the OC was bonded with hydroxyapatite, while in the mice without Esp this was only 74%. This fact raises the possibility that OST-PTP, regulated by the Esp gene, would be in some way involved in the gamma-carboxylation of OC [21].
OC is secreted by the osteoblasts following many posttranslational modifications. These modifications include the excision of a pre-propeptide and gamma-carboxylation, dependent on vitamin K, of the three glutamic residues in the Gla residues [22]. This gamma-carboxylation is essential in order for the protein to have a high mineral affinity and to enable OC to attract calcium ions and incorporate them into the hydroxyapatite crystals, which form 70% of bone [22].
There is a high proportion of non-carboxylated OC in circulation, while a greater proportion of carboxylated OC is found in the bone matrix. However, not all the residues of the three glutamic acids are completely carboxylated and incorporated into the crystals, and the degree of carboxylation varies [23]. Vitamin K is a cofactor for the enzyme glutamate carboxylase which is required for the carboxylation of the proteins which contain Gla in the coagulation cascade and for the carboxylation of OC. Carboxylation or its absence makes OC susceptible to release by the osteoblasts into the circulation. Experiments in vitro carried out in isolated islets and in primary adipocytes, have revealed that the carboxylated form is inactive while the non-carboxylated form is the active form [4,24].
As we have already commented, the (Esp-/-) mice and mice treated with non-carboxylated OC resulted in a protector phenotype, as opposed to the (OC-/-) model. Alternatively, the overexpression of OST-PTP resulted in a phenotype identical to the (OC-/-) mice [4,25].
The metabolic changes in mice with a deletion of the insulin receptor in the osteoblasts provides evidence of a link between the action of the insulin in bone remodelling and glucose homeostasis.
The signalling of the insulin in the osteoblasts inhibits carboxylation, which means that it is a positive regulator of metabolic activity. OST-PTP inhibits insulin signalling, such that the metabolic phenotype of mice without Esp is secondary to an increase in insulin signalling in the osteoblasts and to the secondary increase in non-carboxylated OC [25].
Insulin promotes the ability of osteoblasts to improve bone resorption. The expression of OPG is reduced, through Fox01, and this promotes bone resorption and, in particular, the expression of Tcigr 1 which codes for a proton pump subunit which contributes to the acidification of the extracellular bone matrix [21]. The acidic pH of around 4.5 generated during bone resorption is sufficient to de-carboxylate and activate the molecules of OC (Gla-OCN) stored in the extracellular bone matrix. Sufficient quantities of non-carboxylated OC are produced to induce the expression of the beta cells and to stimulate in them the expression of insulin, just as with treatment with recombinant OC [25]. This shows a pH-dependent activation mechanism for this hormone, and identifies insulin signalling as a critical link between bone remodelling and energy metabolism [25,26].
The data obtained in numerous studies in humans regarding the relationship of OC with glucose homeostasis and, on the one hand, energy metabolism and, on the other, bone metabolism, are highly significant. In the first area, the findings are very interesting. The mice models show OC to be a hormone which positively regulates the production of insulin and its sensitivity. Low levels of OC are associated with diabetes mellitus, glucose intolerance and insulin resistance. Additional studies have established that low levels of OC reduce glycosylated haemoglobin (HbA1c), insulin when fasting and insulin resistance by means of the homeostasis model assessment (HOMA), independently of the presence of diabetes mellitus [6,27-29]. These data suggest that an increase in the levels of OC could be a therapy for the treatment of diabetes in the future.
It has also been found that levels of OC in circulation are inversely correlated with parameters of adiposity, such as body mass index (BMI) or fat mass, and with levels of blood glucose in men and women of different ethnicities [6,20,30].
Levels of circulating OC have also been associated with a number of lipid abnormalities, and are positively correlated with levels of adiponectin in postmenopausal women [20]. More generally, obese people have less OC than those who are not obese, and those with diabetes mellitus type 2 have less OC than non-diabetics [20,30].
The dynamic production of OC as a marker for energy and glucose metabolism has begun to be tested. For example, it has been seen that a significant loss of weight causes a decrease in the homeostasis model assessment for insulin resistance (HOMA-IR), as well as an increase in levels of OC in obese children [31]. In non-diabetics, loss of weight through diet and exercise increases levels of OC in parallel with the decrease in visceral fat, and increases sensitivity to insulin [32,33]. Lower levels of OC have also been correlated with acute myocardial infarction.
In terms of the second area, it has been widely reported that the alterations in glucose metabolism may be a risk factor in loss of bone mass and fractures in humans [34]. In a transverse study it was found that osteoporotic women, after 6 months or more of treatment with alendronate had lower levels of non-carboxylated OC than those who had not been treated. There are scarcely any studies which establish the effects of bisphosphonates on the homeostasis of glucose or insulin sensitivity [35]. Treatment with alendronate or raloxifene (selective estrogen-receptor modulator) for 12 weeks reduced the biochemical markers for bone remodelling, including OC [36].
It would be an important step to confirm whether the antiresorptive drugs, which are the main treatment for osteoporosis, may in some cases have a negative effect on glucose tolerance, as well as the effect of the anticoagulants which affect vitamin K [25].
Lastly, it is also worth highlighting the possible relationship which appears to exist between OC and the endocrine regulation of male reproduction through the regulation of androgen production [37,38].
Conclusions
Recent models in mice suggest a new role for bone in which it is the source of a hormone, non-carboxylated OC, which affects energy metabolism, insulin resistance, obesity and the development of diabetes. So, an organ with an endocrine function, such as fat, is mediated by the endocrine function of another organ, the skeleton. Studies which should be carried out, based on this physiological integration, should review other new areas which have not yet been studied.
There are increasing numbers of studies which are establishing that many aspects of the biology of OC are similar in mice and humans, and which are attempting to determine whether or not the endocrine system of mice is different to that in humans.
The possible role of adiponectin, leptin, OC and insulin, which appear to integrate the functions of the adipose, bone and pancreatic tissues to regulate the metabolism of glucose, bone remodelling, energy and lipid metabolism, remains to be clarified.
We may consider that the skeleton, as an endocrine organ, is involved in the overall co-ordination of energy use, through interaction with other tissues. The identification of OC, and its regulation and different effects, has provided the basis for the development of potential therapeutic applications for metabolic diseases such as diabetes, sarcopenia or obesity.
Both authors declare that there are no conflicts of interest.
![]() Correspondence:
Correspondence:
Marta González-Rozas
c/Miguel Servet s/n
40020 Segovia (Spain)
e-mail: martaglezrozas@yahoo.es
Date of receipt: 23/04/2014
Date of acceptance: 04/07/2014
Bibliography
1. Karsenty G. Convergence between bone and energy homeostases: leptin regulation of bone mass. Cell Metab 2006;4:341-8. [ Links ]
2. Rappaport R. Reciprocal regulation of bone and energy metabolism. Growth Genetics Hormones 2009;25:24-5. [ Links ]
3. Harada SI, Rodan GA. Control of osteoblast function and regulation of bone mass. Nature 2003;423:349-55. [ Links ]
4. Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, et al. Endocrine regulation of energy metabolism by the skeleton. Cell 2007;130:456-69. [ Links ]
5. Wolf G. Energy regulation by the skeleton. Nutr Rev 2008;66:229-33. [ Links ]
6. Pittas AG, Harris SS, Eliades M, Stark P, Dawson-Hughes B. Association between serum osteocalcin and markers of metabolic phenotype. J Clin Endocrinol Metab 2009;94:827-32. [ Links ]
7. Karsenty G, Oury F. The central regulation of bone mass, the first link between bone remodeling and energy metabolism. J Clin Endocrinol Metab 2010;95:4795-801. [ Links ]
8. Ducy P. The role of osteocalcin in the endocrine cross-talk between bone remodelling and energy metabolism. Diabetología 2011;54:1291-7. [ Links ]
9. Wei J, Ducy P. Co-dependence of bone and energy metabolism. Arch Biochem Biophys 2010;503:35-40. [ Links ]
10. Riggs BL, Melton LJ 3rd. Involutional osteoporosis. N Engl J Med 1986;314:1676-86. [ Links ]
11. Zhao LJ, Liu YJ, Yuan Liu P, Hamilton J, Recker RR, Den HW. Relationship of obesity with osteoporosis. J Clin Endocrinol Metab 2007;92:1640-6. [ Links ]
12. Grinspoon S, Thomas E, Pitts S, Gross E, Mickley D, Miller K, et al. Prevalence and predictive factors for regional osteopenia in women with anorexia nervosa. Ann Intern Med 2000;133:790-4. [ Links ]
13. Tremollieres FA, Pouilles JM, Ribot C. Vertebral postmenopausal bone loss is reduced in overweight women: a longitudinal study in 155 early postmenopausal women. J Clin Endocrinol Metab 1993;77:683-6. [ Links ]
14. Rosen CJ. Bone remodeling, energy metabolism, and the molecular clock. Cell Metab 2008;7:7-10. [ Links ]
15. Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, et al. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell 2000;100:197-207. [ Links ]
16. Hauschka PV, Lian JB, Cole DE, Gundberg CM. Osteocalcin and matrix Gla protein: vitamin K-dependent proteins in bone. Physiol Rev 1989;69:990-1047. [ Links ]
17. Murshed M, Schinke T, McKee MD, Karsenty G. Extracellular matrix mineralization is regulated locally; different roles of two gla-containing proteins. J Cell Biol 2004;165:625-30. [ Links ]
18. Dacquin R, Mee PJ, Kawaguchi J, Olmsted-Davis EA, Gallagher JA, Nichols J, et al. Knock-in of nuclear localised beta-galactosidase reveals that the tyrosine collar. Dev Dyn 2004;229:826-34. [ Links ]
19. Kim HY, Choe JW, Kim HK, Bae SJ, Kim BJ, Lee SH, et al. Negative association between metabolic syndrome and bone mineral density in Koreans, especially in men. Calcif Tissue Int 2010;86:350-8. [ Links ]
20. Lee NK. An evolving integrative physiology: skeleton and energy metabolism. BMB Rep 2010;43:579-83. [ Links ]
21. Wah NG. Regulation of glucose metabolism and the skeleton. Clin Endocrinol (Oxf) 2011;75:147-55. [ Links ]
22. Bügel S. Vitamin K and bone health in adult humans. Vitam Horm 2008;78:393-416. [ Links ]
23. Motyl KJ, McCabe LR, Schwartz AV. Bone and glucose metabolism: a two-way street. Arch Biochem Biophys 2010;503:2-10. [ Links ]
24. Lee NK, Karsenty G. Reciprocal regulation of bone and energy metabolism. Trends Endocrinol Metab 2008;19:161-6. [ Links ]
25. Ferron M, Wei J, Yoshizawa T, Del Fattore A, DePinho RA, Teti A, et al. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell 2010;142:296-308. [ Links ]
26. Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell 2000;103:211-25. [ Links ]
27. Hwang YC, Jee JH, Jeong IK, Ahn KJ, Chung HY, Lee MK. Circulating osteocalcin level is not associated with incident type 2 diabetes in middle-aged male subjects: mean 8.4-year retrospective follow-up study. Diabetes Care 2012;35:1919-24. [ Links ]
28. Kanazawa I, Yamaguchi T, Yamamoto M, Yamauchi M, Kurioka S, Yano S, et al. Serum osteocalcin level is associated with glucose metabolism and atherosclerosis parameters in type 2 diabetes mellitus. J Clin Endocrinol Metab 2009;94:45-9. [ Links ]
29. Bao YQ, Zhou M, Zhou J, Lu W, Gao YC, Pan XP, et al. Relationship between serum osteocalcin and glycaemic variability in Type 2 diabetes. Clin Exp Pharmacol Physiol 2011;38:50-4. [ Links ]
30. Kindblom JM, Ohlsson C, Ljunggren O, Karlsson MK, Tivesten A, Smith U, et al. Plasma osteocalcin is inversely related to fat mass and plasma glucose in elderly Swedish men. J Bone Miner Res 2009;24:785-91. [ Links ]
31. Reinehr T, Roth CL. A new link between skeleton, obesity and insulin resistance: relationships between osteocalcin, leptin and insulin resistance in obese children before and after weight loss. Int J Obes (Lond) 2010;34:852-8. [ Links ]
32. Fernández-Real JM, Izquierdo M, Ortega F, Gorostiaga E, Gómez-Ambrosi J, Moreno-Navarrete JM, et al. The relationship of serum osteocalcin concentration to insulin secretion, sensitivity, and disposal with hypocaloric diet and resistance training. J Clin Endocrinol Metab 2009;94:237-45. [ Links ]
33. Levinger I, Zebaze R, Jerums G, Hare DL, Selig S, Seeman E. The effect of acute exercise on undercarboxylated osteocalcin in obese men. Osteoporos Int 2011;22:1621-6. [ Links ]
34. Kemink SA, Hermus AR, Swinkels LM, Lutterman JA, Smals AG. Osteopenia in insulin-dependent diabetes mellitus; prevalence and aspects of pathophysiology. J Endocrinol Invest 2000;23:295-303. [ Links ]
35. Aonuma H, Miyakoshi N, Hongo M, Kasukawa Y, Shimada Y. Low serum levels of undercarboxylated osteocalcin in postmenopausal osteoporotic women receiving an inhibitor of bone resorption. Tohoku J Exp Med 2009;218:201-5. [ Links ]
36. Johnell O, Scheele WH, Lu Y, Reginster JY, Need AG, Seeman E. Additive effects of raloxifene and alendronate on bone density and biochemical markers of bone remodeling in postmenopausal women with osteoporosis. J Clin Endocrinol Metab 2002;87:985-92. [ Links ]
37. Patti A, Gennari L, Merlotti D, Dotta F, Nuti R. Endocrine actions of osteocalcin. Int J Endocrinol 2013;2013:846480. doi: 10.1155/2013/846480. Epub 2013 Apr 30. [ Links ]
38. Schuh-Huerta SM, Pera RA. Reproductive biology: bone returns the favour. Nature 2011;472(7341):46-7. [ Links ]











 texto em
texto em