Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista de Osteoporosis y Metabolismo Mineral
versión On-line ISSN 2173-2345versión impresa ISSN 1889-836X
Rev Osteoporos Metab Miner vol.6 no.4 Madrid nov./dic. 2014
https://dx.doi.org/10.4321/S1889-836X2014000400003
Study of the deletions in the GSTM1 and GSTT1 genes and of the Ile105Val polymorphism of the GSTP1 gene in patients with Paget's disease of bone
Estudio de las deleciones de los genes GSTM1 y GSTT1 y del polimorfismo Ile105Val del gen GSTP1 en pacientes con enfermedad ósea de Paget
Usategui-Martín R.1,2, Corral E.1, Alonso M.1, Calero-Paniagua I.2,3, Carranco-Medina T.E.2,3, Quesada-Moreno A.2,3, Sánchez-González M.D.2,3, Hidalgo-Calleja C.2,3, Pérez-Garrido L.2,3, Montilla Morales C.2,3,4, Mirón-Canelo J.A.5, González-Sarmiento R.1,2,4 and del Pino-Montes J.2,3,4
1 Unidad de Medicina Molecular - Departamento de Medicina - Universidad de Salamanca
2 Instituto de Investigación Biomédica de Salamanca (IBSAL)
3 Servicio de Reumatología - Hospital Universitario de Salamanca
4 Red Temática de Investigación Cooperativa en Envejecimiento y Fragilidad (RETICEF)
5 Departamento de Medicina Preventiva, Salud Pública y Microbiología Médica - Universidad de Salamanca
SUMMARY
Background: Paget's disease of bone (PDB) is a disorder focussed on the bone with an increase in the number, size and activity of the osteoclasts. Some epidemiological data support the theory of its relationship with toxic or infectious environmental agents, whose interaction with some predisposing genetic alterations may lead to PDB. The glutathione S-transferases (GST) are involved in the metabolism of toxins, by catalysing the nucleophilic attack of the physiological substrate, reduced glutathione or GSH (g-Glu-Cys-Gly) on the electrophilic centre of a great number of toxic structures. We studied whether the variability of the GSTM1, GSTP1 and GSTT1 genes is related to the risk of developing PDB.
Patients and methods: We analysed 148 patients diagnosed with PDB, and 207 control individuals matched in sex and age with no history of bone alterations. Using genomic DNA obtained from peripheral blood the presence-absence of the GSTM1 and GSTT1 genes was studied by means of multiplex PCR. The study of the Ile105Val GSTP1 gene was carried out using PCR and subsequent digestion with the restriction enzyme BsmAI. The distribution of genotypes was analysed by means of the Pearson chi-square test. When statistically significant differences were found we carried out a multivariate logistical regression to determine the risk which the presence of a particular genotype could generate. We used the CSPSS 21.0 program. Differences were considered to be statistically significant when the value of p<0.05.
Results: We found differences in the distribution of the presence-absence of the deletion in the GSTM1 gene; not being a carrier for the deletion or being a heterozygous carrier in the GSTM1 gene confers a lower risk of developing PDB (OR=0.56, 95% CI: 0.36-0.87; p=0.011). In the study of the GSTT1 and GSTP1 genes there were no significant differences.
Conclusion: The detoxifying activity diminishes when two copies of the GSTM1 gene with deletions are inherited by reducing in enzyme activity, which has been associated with a greater susceptibility to some cancers, alcoholic hepatopathy and other inflammatory problems. We are not aware of any description of its association with PDB. PDB is observed more frequently in carriers of the homozygous deletion in the GSTM1 gene. This fact could explain the epidemiological findings which link PDB to exposure to certain environmental agents.
Key words: Paget's disease of bone, glutathione S-transferase, genetics, polymorphism.
RESUMEN
Fundamento: La enfermedad ósea de Paget (EOP) es un trastorno focal del hueso con aumento el número, tamaño y actividad de los osteoclastos. Algunos datos epidemiológicos apoyan la teoría de su relación con agentes ambientales tóxicos o infecciosos. Su interacción con algunas alteraciones genéticas predisponentes conducirían a la EOP. Las glutatión-S-transferasas (GST) intervienen en la metabolización de toxinas, al catalizar el ataque nucleofílico del sustrato fisiológico, glutatión reducido o GSH (g-Glu-Cys-Gly) sobre el centro electrófilo de un gran número de estructuras tóxicas. Estudiamos si la variabilidad de los genes GSTM1, GSTP1 y GSTT1 se relaciona con el riesgo a desarrollar EOP.
Pacientes y métodos: Analizamos a 148 pacientes diagnosticados de EOP y a 207 individuos controles pareados en sexo y edad sin antecedentes de alteraciones óseas. Con DNA genómico obtenido de sangre periférica se estudió la presencia-ausencia de deleción en los genes GSTM1 y GSTT1, mediante PCR multiplex. El estudio del polimorfismo Ile105Val del gen GSTP1 se llevó a cabo mediante PCR y posterior digestión con la enzima de restricción BsmaI. Se analizó la distribución de genotipos mediante el test chi-cuadrado de Pearson. Cuando se encontraron diferencias estadísticamente significativas, realizamos una regresión logística multivariante para conocer el riesgo que puede generar la presencia de un determinado genotipo. Utilizamos el programa SPSS 21.0. Se consideraron diferencias estadísticamente significativas aquéllas con valores de p<0,05.
Resultados: Encontramos diferencias en la distribución de la presencia-ausencia de deleción en el gen GSTM1; no ser portador de la deleción o serlo en heterocigosis en el gen GSTM1 confiere un menor riesgo a desarrollar EOP (OR=0,56, IC 95%: 0,36-0,87; p=0,011). En el estudio de los genes GSTT1 y GSTP1 no hubo diferencias significativas.
Conclusión: La actividad detoxificadora disminuye cuando se heredan las dos copias delecionadas del gen GSTM1 al disminuir la actividad enzimática; se ha asociado con una mayor susceptibilidad para algunos tumores, hepatopatía alcohólica y otros problemas inflamatorios. No conocemos descripción de su asociación con la EOP. En los individuos portadores del gen GSTM1 delecionado en homocigosis se observa con más frecuencia EOP. Este hecho podría explicar los hallazgos epidemiológicos que asocian la EOP a la exposición a determinados agentes ambientales.
Palabras clave: enfermedad ósea de Paget, glutation-S-transferasa, genética, polimorfismo.
Introduction
Paget's disease of bone is the most common bone metabolic disease after osteoporosis1. It is a bone disorder characterised by an increase in bone turnover in a disorganised way: a large increase in bone resorption, followed by bone formation of the same proportions. The result is bone with a structure which is irregular and anarchic, which alters its morphology and mechanical properties. Some patients are asymptomatic, while others have pain, degenerative arthropathy, fractures, bone deformities, deafness or other syndromes of nerve compression. The main change resides in the osteoclasts which increase in number size and activity2,3.
There are currently two etiopathogenic hypotheses which attempt to explain the origin of PDB: the influence of environmental factors and the existence of genetic determinants1.
There is evidence that genetic changes play a significant role in the development of the disease. There is a strong tendency to family aggregation (15-40%), with a seven-fold increase in relative risk of suffering the disease in families of patients with PDB3-6. In most of these families there is an autosomal dominant pattern with high penetrance in the sixth decade3. Recently, alterations in the genes SQSTM1, CSF1, OPTN, TNFRSF and TM7SF47,8, have been associated with a higher risk of developing PDB. Some epidemiological data, such as its heterogeneous distribution, or more recent changes in its incidence and seriousness, support the idea of the influence of environmental factors in the development of the disease. Its association with diets poor in calcium and vitamin D during infancy9.10, exposure to environmental toxins11, contact with animals during infancy12-14, consumption of non-controlled meat15, consumption of untreated water16 and infectious agents such as viruses (paramixoviridae)14,17,18, have been reported.
Neither environmental nor genetic factors alone explain the etiopathogeny of this disease. The most accepted model considers PDB to be the result of the synergetic action of both environmental and genetic factors. The genetic determination would explain the individual susceptibility to developing the disease following the exposure to the participating environmental factor2.
Involved in the metabolization of toxins are the glutathione S-transferases (GSTs). These are a family of enzymes which participate in cell detoxification. These enzymes catalyse the nucleophilic attack of the physiological substrate, reduced glutathione or GSH (g-Glu-Cys-Gly) on the electrophilic centres of a great number of toxic structures, allowing their degradation. They are classified in seven families (alpha, kappa, mu, pi, sigma, theta and zeta) which are differentiated both in their sequences, as well as in their immunological properties and physiological roles19,20. GSTM1, GSTP1 and GSTT are the most studied GSTs, and those which have been most commonly associated with human pathologies21.
The role of environmental factors, some of which are toxic, appears to be significant in the development of the disease. Given that the individual response to the toxic factors is genetically determined, we have designed this study with the aim of trying to determine whether the variability of the GSTM1 GSTP1 and GSTT1 genes (involved in the metabolization of exogenous toxins) is related to the risk of developing PDB.
Materials and methods
Patients and controls
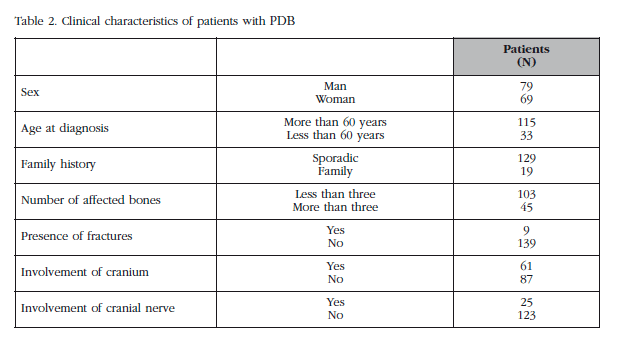
We studied 148 patients diagnosed with PDB. In the case of patients with family history of the disease, we only selected one patient per family to avoid familial genotype bias. The patients were diagnosed by the rheumatology service of the University Hospital of Salamanca. As a control group, we analysed 207 individuals, matched in sex and age with the group of patients, without history of bone alterations, and coming from the same geographic area. From each of the patients were gathered clinical characteristics such as sex, age at diagnosis, family history, number of bones affected, presence of fractures, affectation of skull and affectation of cranial nerve pairs. All the subjects studied, both in the group of patients and the control group, gave their informed consent to participate in the study, which was approved by the ethics committee of the hospital.
DNA extraction and analysis of polymorphisms
In both the patient and control groups the extraction of genomic DNA from peripheral blood was carried out using the standard phenol-chloroform procedure.
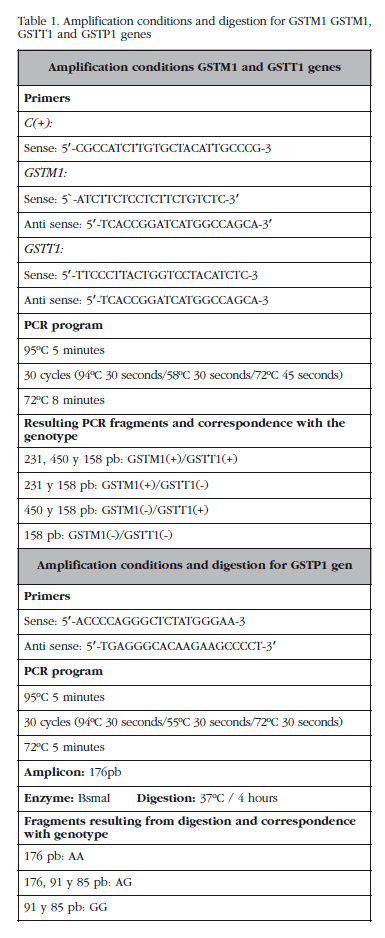
The study of the presence-absence of the deletions in the GSTM1 and GSST1 genes was carried out using multiplex PCR under conditions described in Table 1. The study of the Ile105Val polymorphism of the GSTP1 gene was conducted using PCR and subsequent digestion with the restriction enzyme BsmAl. The conditions used are set out in Table 1.
Statistical analysis
The distribution of genotypes among patients and controls was analysed using the Pearson chi-square test. In those polymorphisms in which statistically significant differences were found we carried out a multivariate logistical regression to determine the risk which the presence of a particular genotype could generate. The statistical analysis was carried out using the SPSS 21.0 program. Those differences whose p value was <0.05 were considered as statistically significant.
Results
We studied a total of 148 patients and 207 controls. The clinical characteristics of the patients are set out in Table 2. The distribution of the presence-absence of deletions in the GSTM1 and GSST1 genes and the distribution of the genotypes for the Ile105Val polymorphism in the GSTP1 gene, and their relationship to the risk of developing PDB are shown in Table 3.
We found statistically significant differences in the distribution of the presence-absence of deletion in the GSTM1 gene: not being a carrier for the homozygous deletion in the GSTM1 gene confers a lower risk of developing PDB (OR=0.56, CI 95%: 0.36-0.87; p=0.011). In the study of the GSTTT1 and GSTP1 genes we found no statistically significant differences (Table 3).
No statistically significant differences were found in the analysis of the clinical characteristics of the patients in relation to the variability of the GSTM1, GSTT1 and GSTP1 genes.
Discussion
PDB lesions occur as the result of an increase in bone resorption followed by an increase in its formation. The main change is located in the osteoclasts which increase in number, size and activity. There is a range of evidence which indicates that the etiopathogeny of the disease is a synergy between a series of environmental factors and the existence of certain genetic determinants2. Through a study of the variability of the GSTM1, GSTT1 and GSTP1 genes (involved in the metabolism of endogenous toxins) we intended to evaluate the relationship between these variables and the risk of developing PDB. As far as we know, this is the first work which examines the influence of the changes in these genes on the development of this disease.
The GSTM gene is located in the 1p13 chromosome, and to date, five allelic variants are known: GSTM1, GSTM2, GSTM3, GSTM4 and GSTM5. A reduction in detoxification activity occurs when the deletion in gene GSTM1 is inherited, meaning that being a homozygous carrier of a deletion in the GSTM1 gene causes a reduction in enzyme activity. The theta class of GSTs comprises two genes which code for the two proteins GSTT1 and GSTT2. As with the GSTM1 gene, if a homozygous deletion in the GSTT1 gene is inherited, there is a reduction in detoxification activity. In terms of the sub-family of GSTP, it comprises a single gene GSTP1 in which have been described two allelic variants which differ in the base 313 of the cDNA, one adenine (A) being substitute by a guanine (G). This difference results in a change of a valine (Val) to an isoleucine (Ile) in the 105 codon of the amino-acid sequence, causing a defective bond between the enzyme and the substrate, and thus, a reduction detoxification activity19,20,22,23.
Being a homozygous carrier for deletion in the GSTM1 and/or GSTT1 genes has been associated with a greater susceptibility to developing different types of cancer21,22,24, alcohol-related liver disease25 and other inflammatory diseases25,26, because it causes poorer metabolization of toxic agents, with the synthesis of free radicals which damage DNA20. Our results show that not being a homozygous carrier for the deletion in the GSTM1 gene brings a lower risk of suffering PDB. In the study of the GSTT1 and GSTP1 genes we found no statistically significant difference between the patient and control groups. We found no statistically significant differences between the clinical expression, extent and activity of the disease in relation to the variability in the GSTM1, GSTT1 and GSTP1 genes in the group of patients with PDB.
One of the causes postulated as the origin of PDB is exposure to environmental toxins from the production of cotton, meat or drinking water without adequate control of sanitation, which may alter the maturation and activity of the osteoclasts, the increase in activity fostering the development of PDB11,15,16. Our hypothesis is that to have the homozygous deletion in the GSTM1 gene assumes poor metabolization of environmental toxins which, by a mechanism yet unknown, may increase the function of the osteoclasts and osteoclast precursors which, combined with other genetic changes not yet well described, could result in the development of PDB.
In conclusion, in those individuals who are carriers of the GSTM1 gene with homozygous deletions, PDB is more frequently observed.This fact could explain the epidemiological findings which associate PDB with exposure to certain environmental agents. Even so, functional studies of these polymorphisms are required in order to validate our hypothesis.
![]() Correspondence:
Correspondence:
Javier del Pino Montes
Servicio de Reumatología
Hospital Universitario de Salamanca
Po San Vicente, 182
37007 Salamanca (Spain)
E-mail: jpino@usal.es
Date of receipt: 29/08/2014
Date of acceptance: 23/11/2014
Bibliography
1. Ralston SH, Layfield R. Pathogenesis of Paget Disease of Bone. Calcif Tissue Int 2012;91:97-113. [ Links ]
2. Singer FR, Mills BG, Gruber HE, Windle JJ, Roodman GD. Ultrastructure of bone cells in Paget's disease of bone. J Bone Miner Res 2006;21(Suppl 2):P51-4. [ Links ]
3. Morales-Piga AA, Rey-Rey JS, Corres-González J, García-Sagredo JM, López-Abente G. Frequency and characteristics of familial aggregation of Paget's disease of bone. J Bone Miner Res 1995;10:663-70. [ Links ]
4. Morissette J, Laurin N, Brown JP. Sequestosome 1: mutation frequencies, haplotypes, and phenotypes in familial Paget's disease of bone. J Bone Miner Res 2006;21(Suppl 2):P38-44. [ Links ]
5. Siris ES, Ottman R, Flaster E, Kelsey JL. Familial aggregation of Paget's disease of bone. J Bone Miner Res 1991;6:495-500. [ Links ]
6. Hocking LJ, Herbert CA, Nicholls RK, Williams F, Bennett ST, Cundy T, et al. Genomewide search in familial Paget disease of bone shows evidence of genetic heterogeneity with candidate loci on chromosomes 2q36, 10p13, and 5q35. Am J Hum Genet 2001;69:1055-61. [ Links ]
7. Albagha OM, Visconti MR, Alonso N, Langston AL, Cundy T, Dargie R, et al. Genome wide association study identifies variants at CSF1, OPTN and TNFRSF11A as genetic risk factors for Paget's disease of bone. Nat Genet 2010;42:520-4. [ Links ]
8. Albagha OME, Wani SE, Visconti MR, Alonso N, Goodman K, Brandi ML, et al. Genome-wide association identifies three new susceptibility loci for Paget's disease of bone. Nat Genet 2011;43:685-9. [ Links ]
9. Barker DJ, Gardner MJ. Distribution of Paget's disease in England, Wales and Scotland and a possible relationship with vitamin D deficiency in childhood. Br J Prev Soc Med 1974;28:226-32. [ Links ]
10. Siris ES. Epidemiological aspects of Paget's disease: family history and relationship to other medical conditions. Semin Arthritis Rheum 1994;23:222-5. [ Links ]
11. Lever JH. Paget's disease of bone in Lancashire and arsenic pesticide in cotton mill wastewater: a speculative hypothesis. Bone 2002;31:434-6. [ Links ]
12. Merlotti D, Gennari L, Galli B, Martini G, Calabrò A, De Paola V, et al. Characteristics and familial aggregation of Paget's disease of bone in Italy. J Bone Miner Res 2005;20:1356-64. [ Links ]
13. López-Abente G, Morales-Piga A, Elena-Ibáñez A, Rey-Rey JS, Corres-González J. Cattle, pets, and Paget's disease of bone. Epidemiology 1997;8:247-51. [ Links ]
14. O'Driscoll JB, Anderson DC. Past pets and Paget's disease. Lancet 1985;2:919-21. [ Links ]
15. Mills BG, Singer FR. Nuclear inclusions in Paget's disease of bone. Science 1976;194:201-2. [ Links ]
16. Mirón-Canelo JA, Del Pino-Montes J, Vicente-Arroyo M, Sáenz-González MC. Epidemiological study of Paget's disease of bone in a zone of the Province of Salamanca (Spain). The Paget's disease of the bone study group of Salamanca. Eur J Epidemiol 1997;13:801-5. [ Links ]
17. Rebel A, Malkani K, Basle M, Bregeon C, Patezour A, Filmon R. Ultrastructural characteristics of osteoclasts in Paget's disease. Rev Rhum Mal Osteoartic 1974;41:767-71. [ Links ]
18. Mills BG, Singer FR, Weiner LP, Suffin SC, Stabile E, Holst P. Evidence for both respiratory syncytial virus and measles virus antigens in the osteoclasts of patients with Paget's disease of bone. Clin Orthop Relat Res 1984:303-11. [ Links ]
19. Strange RC, Spiteri MA, Ramachandran S, Fryer AA. Glutathione-S-transferase family of enzymes. Mutat Res 2001;482:21-6. [ Links ]
20. Strange RC, Jones PW, Fryer AA. Glutathione S-transferase: genetics and role in toxicology. Toxicol Lett 2000;112-113:357-63. [ Links ]
21. Parl FF. Glutathione S-transferase genotypes and cancer risk. Cancer Lett 2005;221:123-9. [ Links ]
22. Ye Z, Song H. Glutathione s-transferase polymorphisms (GSTM1, GSTP1 and GSTT1) and the risk of acute leukaemia: a systematic review and meta-analysis. Eur J Cancer 2005;41:980-9. [ Links ]
23. Frova C. Glutathione transferases in the genomics era: New insights and perspectives. Biomol Eng 2006;23:149-69. [ Links ]
24. White DL, Li D, Nurgalieva Z, El-Serag HB. Genetic variants of glutathione S-transferase as possible risk factors for hepatocellular carcinoma: a HuGE systematic review and meta-analysis. Am J Epidemiol 200815;167:377-89. [ Links ]
25. Brind AM, Hurlstone A, Edrisinghe D, Gilmore I, Fisher N, Pirmohamed M, et al. The role of polymorphisms of glutathione S-transferases GSTM1, M3, P1, T1 and A1 in susceptibility to alcoholic liver disease. Alcohol Alcohol 2004;39:478-83. [ Links ]
26. Miller EA, Pankow JS, Millikan RC, Bray MS, Ballantyne CM, Bell DA, et al. Glutathione-S-transferase genotypes, smoking, and their association with markers of inflammation, hemostasis, and endothelial function: the atherosclerosis risk in communities (ARIC) study. Atherosclerosis 2003;171:265-72. [ Links ]











 texto en
texto en