My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista de Osteoporosis y Metabolismo Mineral
On-line version ISSN 2173-2345Print version ISSN 1889-836X
Rev Osteoporos Metab Miner vol.6 suppl.1 Madrid Mar. 2014
https://dx.doi.org/10.4321/S1889-836X2014000500001
How to use vitamin D, and what supplementary dose would be the optimum to achieve the best balance between efficacy and security?
¿Cómo utilizar la vitamina D y qué dosis de suplementación sería la más idónea para tener el mejor balance eficacia/seguridad?
Torres del Pliego E., Nogués Solán X.
Servicio Medicina Interna - Unidad de Investigación en Fisiopatología Osea y Articular (URFOA) - Instituto Hospital del Mar de Investigaciones Médicas (IMIM) - Red de Envejecimiento y Fragilidad (RETICEF) - Universidad Autónoma de Barcelona (UAB)
Vitamin D is a steroid synthesised in the skin by exposure to sunlight and/or through ingestion of foods which contain it, and it plays a fundamental role in the mineralisation of the bone system at all ages. Vitamin D is not only a nutrient but is also considered to be a true hormone with various functions, a principal one of which is to maintain blood calcium at a physiologically acceptable level for it to carry out its metabolic functions, the transduction of signals and neuromuscular activity [1].
The process of synthesis and metabolisation of vitamin D has been well known since the 1920s. In summary, the process is initiated by the transformation of 7-dihydrocholsterol into provitamin D and subsequently to vitamin D, initially inert, which then requires two hydroxylations to become biologically active (Figure 1). The first hydroxylation, whether it is of vitamin D2 (ergocalciferol) or vitamin D3 (colecalciferol), takes place in the liver, where it becomes bonded to the binder protein of vitamin D, which turns into 25 (OH) vitamin D, its main circulating form, and whose blood levels are those used to evaluate its state of deficit, normality or intoxication. The second hydroxylation happens mainly and essentially in the kidney - although there are other tissues in which it is also produced, such as the breast, colon, prostate, etc. - where it converts into the biologically active form, 1,25 (OH)2 vitamin D, or calcitriol, whose essential functions are to increase the absorption of calcium and phosphorus in the intestine, inhibit the formation of osteoclasts for bone reabsorption and to reduce the production of the parathyroid hormone (PTH) [2]. But in addition, the 1,25 (OH)2 vitamin D produced locally in tissues not related to calcium metabolism may have the aim of regulating a wide variety of biological functions, including cell growth, apoptosis, angiogenesis, the differentiation and regulation of the immune system, which would be called the non-classical actions of vitamin D. Thus, given that this vitamin participates in no end of physiological functions, an association between a deficit of vitamin D and many acute and chronic diseases, including alterations in the metabolism of calcium, some cancers, type 2 diabetes, cardiovascular disease and infectious diseases [3] has been confirmed.
Optimum levels of vitamin D
Considering the many and important functions of vitamin D both in the skeleton and outside it, it seems logical to assume that the levels of this vitamin need to be optimum for it to be able to fulfil its functions [4]. However, there is still much controversy as to what are the optimum levels of 25(OH) vitamin D for the maintenance of bone health and to reduce the risk of its deficit.
Understanding what are the optimum levels of 25(OH) vitamin D is the starting point for knowing what would be the supplementary intake of vitamin D necessary to reach these levels, given that it is widely known that there is a general deficiency in vitamin D in the population [5,6].
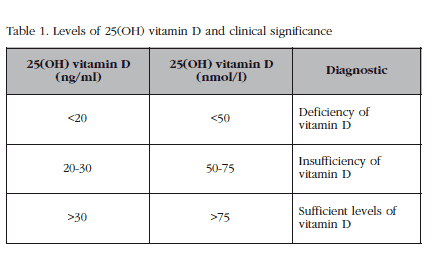
In general, the common understanding of the experts and most of the scientific societies concerned with this matter is to consider a deficiency of vitamin D to be at values lower than 20 ng/ml, insufficiency at between 21-29 ng/ml and sufficiency at values >30 ng/ml, with the a range of 40-16 ng/ml being preferred, and vitamin D intoxication in general, at values higher than 150 ng/ml (Table 1) [7-9]. By general agreement, values below 20 ng/ml are insufficient. However, the argument centres on whether it is necessary to reach 30 ng/ml to achieve vitamin D’s effects in and outside the bone [9]. There are histomorphometric data which indicate that at levels below 30 ng/ml the osteoid volume would be higher, and biopsy data for which there would be a diagnosis of osteomalacia in 25% in individuals with these levels of vitamin D [10].
In the bone, levels of between 24 ng/ml and 32 ng/ml appear to be sufficient to reduce the risk of fracture and even of falls [11].
How to achieve these optimum levels of vitamin D?
The primary and most important source is exposure to sunlight, from which is obtained 90% of vitamin D, whose production depends on the particular angle of the sun. Thus, for example, it has been observed that exposure to the sun of the whole body with minimum erythema (reddening of the skin 24 hours after exposure to the sun) results in the achievement of levels of vitamin D comparable to taking 10,000 to 25,000 UI of vitamin D orally [12]. However, exposure to sunlight in winter at certain latitudes does not produce any vitamin D3 [13]. Hence, in certain places such as Boston, it is recommended that white men and women should expose their face and arms or arms and legs to sunlight approximately three times a week for 25% of the duration which would produce slight sunburn in spring, summer and autumn [12,13]. Other factors which influence reduction in the production of vitamin D by exposure to sunlight are sun protection creams, greater skin pigmentation and older age (essentially older than 65 years) [14,15].
Dietary sources contribute to the achievement of optimum levels of vitamin D. The number of foods which naturally contain a significant quantity of vitamin D is limited, which meant that in Britain in the 1930s some of these, for example, milk, fizzy drinks, bread and even beer, were enriched with vitamin D. However, apparent cases of vitamin D intoxication occurred in children in the 1950s, which resulted in much tighter European regulation, which only permitted the enrichment of margarine, something which continues to the present day. On the other hand, in the United States, since 2003 juices have been enriched, which seems to have had a similar degree of effectiveness as oral supplements [3]. The recommended daily intake of vitamin D is currently the subject of argument. The US Institute of Medicine (IOM), the endocrinology societies and the Task Force are not in agreement as to the necessary daily amount, although they agree that there is a deficiency in the population. The coherent explanation of this matter would be that the IOM and the Task Force have made recommendations for the general healthy population, while the medical societies have tried to make recommendations for patients and special cases [16,17]. This situation has created confusion among staff who are not experts in the field, and editorial arguments between the experts themselves [18].
The IOM recommends 600 UI/day for the population between 1 and 70 years of age and 800 UI/day for the population of 71 years or older, and with a maximum level of daily intake of 4,000 UI to maintain levels of 25(OH) vitamin D above 20 ng/ml, which would be necessary for the general health of the population [19]. On the other hand, the US Society for Endocrinology would recommend levels higher than 30 ng/ml, which means a daily intake of 1,500-2,000 [20].
Along the same lines, the IOF (International Osteoporosis Foundation) also recommends levels higher than 30 ng/ml (75 nmol/L) of 25(OH) vitamin D, requiring, to reach this threshold, supplements of between 800 and 1,000 UI/day (20-25 µg/day). Furthermore, it was established that there is a correlation between the quantity of vitamin D supplements and blood level of 25(OH) vitamin D reached, which would be approximately 2.5 nmol/L (range 1.75-2.75 nmol/L) for each 100 UI (2.5 µg) of additional vitamin D [21]. You would think, therefore, that supplementation in the upper range of the recommendations of IOF (1,000 IU/day) would increase the likelihood of patients achieving levels of 30 ng/ml, compared with a lower dose supplementation. Like the Endocrine Society, the IOF also considers that supplementation with vitamin D could reach 2,000 UI/day in certain patients, among others, obese or osteoporotic people, those with limited exposure to sun (for example institutionalised people), those with problems of absorption, etc [21].
In relation to the above, in terms of bone in general, doses higher than 800 UI would be sufficient to reduce hip and non-vertebral fractures in patients over 65 years of age [22].
Excess or intoxication by vitamin D
The administration of excessive doses of vitamin D does not carry greater benefits, but does have a higher risk of intoxication. The risk of intoxication is determined by the levels of 25(OH) vitamin D and the presence of hypercalcemia. It is certain that intoxication would probably require a dose much higher than 4,000 UI/day. Thus, an excessive intake of vitamin D (normally >10,000 UI/day) over many months may provoke vitamin D intoxication, which is detected by notably raised levels of 25(OH) vitamin D, hypercalcemia and hyperphosphatemia. However, regimens with high doses (of 5,500 to 11,000 UI daily for more than 20 weeks)) in patients with low baseline deficits without hypercalcemia occurring have been described [23].
In obese patients it has been seen that the necessity for vitamin D supplements are greater, which is why some authors have suggested the following formula:
Necessary dose of vitamin D in UI = [weight x desired change in 25(OH) x 2.5] - 10 [24].
However, there are various studies which suggest that there is a non-linear relationship between 25(OH) vitamin D and mortality [25], with an increase at both low and high blood levels of 25(OH) vitamin D. Thus, Melamed et al. [26] found an increase in the risk of all causes of mortality in women with doses of 25(OH) vitamin D <20 ng/ml, but also if >50 ng/ml. A U curve phenomenon appears evident in relation to levels of mortality and vitamin D. Michaëlsson et al. [27] observed 50% higher total mortality in 1,194 males with an average age of 71 years with low levels of 25(OH) vitamin D (around 18.5ng/ml), but also with higher levels (around 39 ng/ml). A recent meta-analysis has shown similar data, with benefits in terms of optimum values of mortality at between 31 and 35 ng/ml [28].
Contrary to what one might assume, there is no unanimous position on the maximum level of vitamin D to avoid an alleged risk. Therefore, controlled randomised studies are required, with the administration of different doses of vitamin D to establish with certainty what are the optimum doses and what doses may become harmful [18,28].
Conclusion
In clinical practice, taking decisions about treatment is always difficult, and even more so when there is disagreement about when and how. At present, the evidence and the consensuses, the guides of the scientific societies and the opinions of the experts show the importance of vitamin D as a hormone which has an influence on numerous metabolic processes, among which, bone metabolism is the most important. All are in agreement that there is a deficit in the general population and, above all, in the population affected by osteoporosis. However, views on the optimum levels of vitamin D may be torn between 20 and 39 ng/ml, although it seems that there is enough consensus in establishing a minimum level of 30 ng/ml. Nor is there unanimity regarding the maximum level which should be reached and if this could be quite dangerous, not in producing serious metabolic alterations such as hypercalcemia, but due to possible increases in cardiovascular mortality.
In terms of supplements, there is agreement on their necessity but the doses are also the subject of argument. It seems clear that for the majority of individuals, between 800 and 1,000 UI a day would be necessary and that a supplement in the range higher than this interval (1,000 UI/day) would increase the probability that the patients would achieve blood levels of 25(OH) vitamin D higher than 30 ng/ml, although it is also possible that some special groups require even higher doses (up to 2,000 UI/day) to reach these levels.
![]() Correspondence:
Correspondence:
Xavier Nogués
Passeig Marítim, 25-29
08003 Barcelona (España)
Correo electrónico: xnogues@hospitaldelmar.cat
Bibliography
1. Holick MF. Vitamin D: A millenium perspective. J Cell Biochem 2003,88:296-307. [ Links ]
2. Girgis CM, Clifton-Bligh RJ, Hamrick MW, Holick MF, Gunton JE. The roles of vitamin D in skeletal muscle: form, function, and metabolism. Endoc Rev 2013;34:33-83. [ Links ]
3. Wacker M, Holick MF. Vitamin D - effects on skeletal and extraskeletal health and the need for supplementation. Nutrients 2013;5:111-48. [ Links ]
4. Mithal A, Wahl DA, Bonjour JP, Burckhardt P, Dawson-Hughes B, Eisman JA, et al. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos Int 2009;20:1807-20. [ Links ]
5. Heaney RP. Vitamin D, nutritional deficiency, and the medical paradigm. J Clin Endocrinol Metab 2003;88:5107-8. [ Links ]
6. Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc 2006;81:353-73. [ Links ]
7. Holick MF, Siris ES, Binkley N, Beard MK, Khan A, Katzer JT, et al. Prevalence of Vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab 2005;90:3215-24. [ Links ]
8. Alshahrani F, Aljohani N. Vitamin D: deficiency, sufficiency and toxicity. Nutrients 2013;5:3605-36. [ Links ]
9. Heaney RP. Health is better at serum 25(OH)D above 30 ng/mL. J Steroid Biochem Mol Biol 2013;136:224-8. [ Links ]
10. Priemel M, von Domarus C, Klatte TO, Kessler S, Schlie J, Meier S, et al. Bone mineralization defects and vitamin D deficiency: histomorphometric analysis of iliac crest bone biopsies and circulating 25-hydroxyvitamin D in 675 patients. J Bone Miner Res 2010;25:305-12. [ Links ]
11. Bischoff-Ferrari HA, Shao A, Dawson-Hughes B, Hathcock J, Giovannucci E, Willett WC. Benefit-risk assessment of vitamin D supplementation. Osteoporos Int 2010;21:1121-32. [ Links ]
12. Holick MF, Chen TC, Lu Z, Sauter E. Vitamin D and skin physiology: a D-lightful story. J Bone Miner Res 2007;22:V28-33. [ Links ]
13. Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab 1988;67:373-8. [ Links ]
14. Tangpricha V, Turner A, Spina C, Decastro S, Chen TC, Holick MF. Tanning is associated with optimal vitamin D status (serum 25-hydroxyvitamin D concentration) and higher bone mineral density. Am J Clin Nutr 2004;80:1645-9. [ Links ]
15. Matsuoka LY, Ide L, Wortsman J, MacLaughlin JA, Holick MF. Sunscreens suppress cutaneous vitamin D3 synthesis. J Clin Endocrinol Metab 1987;64:1165-8. [ Links ]
16. Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011;96:1911-30. [ Links ]
17. Rosen CJ, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, et al. IOM committee members respond to Endocrine Society vitamin D guideline. J Clin Endocrinol Metab 2012;97:1146-52. [ Links ]
18. Bouillon R, Van Schoor NM, Gielen E, Boonen S, Mathieu C, Vanderschueren D, et al. Optimal vitamin D status: a critical analysis on the basis of evidence-based medicine. J Clin Endocrinol Metab 2013;98:E1283-304. [ Links ]
19. Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 2011;96:53-8. [ Links ]
20. Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Guidelines for preventing and treating vitamin D deficiency and insufficiency revisited. J Clin Endocrinol Metab 2012;97:1153-8. [ Links ]
21. Dawson-Hugues B, Mithal A, Bonjour JP, Boonen S, Burckhardt P, Fuleihan EH, Josse RG, Lips P, Morales-Torres J, Yoshimura N. IOF position statement: vitamin D recommendations for older adults. IOF position statement: vitamin D recommendations for older adults. Osteoporos Int 2010 Jul;21:1151-4. [ Links ]
22. Bischoff-Ferrari HA, Willett WC, Orav EJ, Lips P, Meunier PJ, Lyons RA, et al. A pooled analysis of vitamin D dose requirements for fracture prevention. N Engl J Med 2012;367:40-9. [ Links ]
23. Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr 2003;77:204-10. [ Links ]
24. Drincic A, Fuller E, Heaney RP, Armas LA. 25-hydroxyvitamin D Response to Graded Vitamin D3 Supplementation Among Obese Adults. J Clin Endocrinol Metab 2013 Sep 13 (Epub ahead of print). [ Links ]
25. Amer M, Qayyum R. Relationship between 25-hydroxyvitamin D and all-cause and cardiovascular disease mortality. Am J Med 2013;126:509-14. [ Links ]
26. Melamed ML, Michos ED, Post W, Astor B. 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med 2008;168:1629-37. [ Links ]
27. Michaelsson K, Baron JA, Snellman G, Gedeborg R, Byberg L, Sundstrom J, et al. Plasma vitamin D and mortality in older men: a community-based prospective cohort study. Am J Clin Nutr 2010;92:841-8. [ Links ]
28. Zittermann A, Iodice S, Pilz S, Grant WB, Bagnardi V, Gandini S. Vitamin D deficiency and mortality risk in the general population: a meta-analysis of prospective cohort studies. Am J Clin Nutr 2012;95:91-100. [ Links ]











 text in
text in