My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista de Osteoporosis y Metabolismo Mineral
On-line version ISSN 2173-2345Print version ISSN 1889-836X
Rev Osteoporos Metab Miner vol.8 n.4 Madrid Oct./Dec. 2016
ORIGINAL
Effect of RANK/RANKL/OPG pathway on bone demineralization and vascular calcification in chronic kidney disease
Efecto del sistema RANK/RANKL/OPG sobre la desmineralización ósea y la calcificación vascular en la enfermedad renal crónica
Martínez Arias L.1, Solache Berrocal G.1, Panizo García S.1, Carrillo López N.1, Avello Llano N.2, Quirós Caso C.2, Naves Díaz M.1 and Cannata Andía J.B.1
1 Servicio de Metabolismo Óseo y Mineral - Instituto Reina Sofía de Investigación Nefrológica - Red de Investigación Renal (REDinREN) del Instituto de Salud Carlos III - Universidad de Oviedo - Oviedo (España)
2 Laboratorio de Medicina - Hospital Universitario Central de Asturias - Oviedo (España)
This work was made possible thanks to the funding obtained by the AMGEN-SEIOMM 2010 grant to promote research. This work has also been partially financed with the help of the National Plan for R & D & I 2008-2011, State Plan for R & D & I 2013-2016, Carlos III Health Institute (ISCIII) - European Regional Development Fund 09/00415, PI 10/0896 and PI13/00014), Science, Technology and Innovation Plan 2013-2017 of the Principality of Asturias (GRUPIN14-028), Foundation for the Promotion in Asturias of Applied Scientific Research and Technology (FICYT ), Reina Sofía Institute for Nephrology Research, Inigo Álvarez de Toledo Renal Foundation, RETIC RedInRen of the ISCIII - European Regional Development Fund (RD06/0016/1013, RD12/0021/1023 and RD16/0009), by the Asturian Society for Metabolic Research.
Work awarded a scholarship Research AMGEN-SEIOMM 2010.
SUMMARY
Introduction: In cases of chronic kidney disease (CKD), bone and mineral metabolism changes occur which favor soft tissue calcification. Alterations in the RANK/RANKL/OPG system could also favor vascular calcification, a major cause of morbidity and mortality in CKD.
Objective: In an in vivo experimental model of chronic renal failure progression, we assess the effect of CKD on vascular calcification and bone loss correlating these changes in the RANK/RANKL/OPG pathway. An In vitro system was used to confirm findings.
Material and Methods: Two models of vascular calcification were used: an in vivo rat model with chronic renal failure fed on a diet with different phosphorus content, and an In vitro model in vascular smooth muscle cells (VSMC) subjected to different calcifying stimuli.
Results: At 20 weeks, 50% of animals with a diet high in phosphorus presented aortic calcification accompanied by increased aortic expression of RANKL. In contrast, OPG decreased probably as a consequence of an inflammatory component.
At 20 weeks, expression of RANKL and OPG in the tibia increased, while the increase in OPG occurred at earlier stages.
In VSMC, the addition of uremic serum and calcification medium increased calcium content and expression of RANKL and OPG. The addition of OPG and silencing of RANK inhibited this increase.
Conclusions: Our results confirm RANK/RANKL/OPG system involvement in the vascular calcification process.
Key words: RANK, RANKL, OPG, chronic kidney disease, vascular calcification.
RESUMEN
Introducción: En la enfermedad renal crónica (ERC) se producen alteraciones del metabolismo óseo y mineral que favorecen la calcificación de tejidos blandos. Alteraciones del sistema RANK/RANKL/OPG podrían estar favoreciendo la calcificación vascular, importante causa de morbimortalidad en la ERC.
Objetivo: Valorar en un modelo experimental in vivo de insuficiencia renal crónica el efecto de la progresión de la misma sobre la calcificación vascular y sobre la pérdida de hueso correlacionando estos cambios con alteraciones en el sistema RANK/RANKL/OPG, utilizando un sistema In vitro para confirmar los hallazgos encontrados.
Material y métodos: Se utilizaron dos modelos de calcificación vascular: un modelo in vivo en ratas con insuficiencia renal crónica alimentadas con dieta con diferente contenido en fósforo, y un modelo In vitro en células de músculo liso vascular (CMLV) sometidas a diferentes estímulos calcificantes.
Resultados: A las 20 semanas, un 50% de los animales con dieta alta en fósforo presentó calcificaciones aórticas que se acompañó de aumento en la expresión aórtica de RANKL. Por el contrario, la OPG disminuyó como consecuencia probablemente del componente inflamatorio.
A las 20 semanas en la tibia aumentó la expresión de RANKL y OPG, mientras que el aumento de OPG ocurrió en fases más tempranas.
En CMLV la adición de suero urémico y medio calcificante indujo un incremento del contenido de calcio y de la expresión de RANKL y OPG. La adición de OPG y el silenciamiento de RANK inhibieron este aumento.
Conclusiones: Nuestros resultados confirman la participación del eje RANK/RANKL/OPG en el proceso de calcificación vascular.
Palabras clave: RANK, RANKL, OPG, enfermedad renal crónica, calcificación vascular.
Introduction
Vascular calcification is a process in which vascular smooth muscle cells (VSMC) and other populations of blood vessel cells undergo a transformation and begin to resemble osteoblasts1. This process is regulated in a manner similar to bone mineralization, with several bone proteins being implicated2-4. Osteoblasts are cells responsible for the formation of bone that also regulate the activity of osteoclasts and therefore play an important role in the homeostasis of calcium (Ca) and phosphorus (P)5. Osteoblasts secrete the NF-κB activator receptor ligand (RANKL) that binds to its receptor (RANK) in osteoclast precursors promoting formation, activation and survival6-7. In addition, osteoblasts secrete osteoprotegerin (OPG), which acts as a soluble receptor lure of RANKL and inhibits the binding of this ligand to its transmembrane receptor RANK. There is considerable scientific evidence linking the RANK/RANKL/OPG system to vascular calcifications, which may be an important autocrine/paracrine system involved in the process. The pathway by which RANKL promotes calcification through binding to its RANK receptor with the consequent activation of the NF-κB alternative pathway and bone morphogenetic protein 4 (BMP4)8 has been implicated in the osteogenic transition of VSMCs9-10.
Chronic kidney disease (CKD) is characterized by changes in bone and mineral metabolism that favor the calcification of soft tissues and vessels. Alterations in the gene expression of the RANK/RANKL/OPG system could be favoring vascular calcification, one of the main causes of mortality in CKD. It is interesting to investigate the differences in the regulation of the RANK/RANKL/OPG system in bone and vessel in order to design strategies aimed at protecting the bone without having negative effects on vascular calcification.
Therefore, this study aims: a) to evaluate in a rat model the effect of CKD and diets with different P content on vascular calcification quantified by Ca content analysis and bone mineral density (BMD), quantified by bone densitometry; B) to correlate these changes with alterations in the RANK/RANKL/OPG system gene expression in arteries and bones of these animals; And c) to use an in vitro system to confirm the findings found in vivo.
Materials and methods
In vivo studies:
Vascular calcification model
The protocol was approved by the University of Oviedo's Ethical Committee of Animal Experimentation.
The study was performed with male Wistar rats (n=55) at 4 months of age (350-400 g). Surgical intervention, following inhalation of isoflurane anesthesia, involved inducing chronic renal failure (CRF) (7/8) in a single surgical procedure. Complete nephrectomy of the right kidney and then subtotal nephrectomy of the left kidney were carried out by lateral incision. This procedure preserves approximately one fourth of the renal mass. The rats with CRF were divided into two groups: one, CRF C, fed a standard rodent diet with normal P content (0.6% P, 0.6% Ca, and 20% protein content, Panlab, Barcelona, Spain), and the other, CRF P, fed a diet with high P content (0.9% P, 0.6% Ca, and 20% protein content, Panlab). The study lasted 20 weeks (CRF 20C and CRC 20P), time required to induce vascular calcifications. We also included a Sham group (n=10) that was followed up to week 20. Intermediate evaluations were also performed throughout the study, with sacrifices at 8 and 12 weeks (CRF 8C, CRI 12C, CRI 8P and CRI 12P). Twenty-four hours before slaughter, the rats were housed in metabolic cages and received diet and water ad libitum. They were sacrificed using CO2 anesthesia, and serum samples were taken for analysis. From each rat the abdominal aorta was removed down to the bifurcation of the iliac crests and divided into three portions: the first fragment was used for the extraction of RNA, the second fragment to determine the Ca content, and the third fragment was stored in paraffin for future studies.
At the time of sacrifice the two tibia were removed. The left was preserved in alcohol to measure bone mineral density (BMD). The remaining tibia was frozen at -80oC until processed for the study of gene expression.
Biochemical markers
Serum urea, creatinine, Ca and P were measured using a Hitachi 717 multi-channel automatic analyzer (Boehringer Mannheim, Berlin, Germany). Parathyroid hormone (PTH) was measured by ELISA (Immutopics, San Juan Capristano, USA) following the manufacturer's protocol.
Bone densitometry
BMD was measured in tibia at three levels: proximal octave, seven/eighth distal and total tibia, with a Hologic QDR-1000 dual-energy digital radiological densitometer (Hologic, Bedford, USA) equipped with a specific program for small animals.
Analysis of aortic calcification
Calcification of the rats' abdominal aorta was analyzed by two methods: total Ca content and von Kossa staining.
To determine total Ca content, a fragment of the abdominal aorta (the cm proximal to the iliac bifurcation) was homogenized with an Ultraturrax (OmniHT) in 0.6 N HCl. After shaking at 4oC for 24 hours the samples were centrifuged. The Ca content was determined in the supernatant by the o-cresolphthalein complexone method (Sigma-Aldrich, St. Louis, USA), and the pellet was resuspended in lysis buffer (125 mM Tris and 2% SDS , PH 6.8) for protein extraction and quantification by the method of Lowry (Bio-Rad, Hercules, USA). The Ca content was normalized by expressing as µg Ca per mg protein.
To carry out von Kossa staining, another fragment of the abdominal aorta was included in methyl methacrylate (Sigma-Aldrich). Five 5 mm thick sections were obtained using a Polycut S Microtome (Reicher-Jung, Heildelberg, Germany) and stained following the von Kossa method.
Gene expression study
RNA extraction was carried out by the guanidinium-phenol-chloroform thiocyanate method. DNA copy (cDNA) was synthesized using the high capacity kit (Applied Biosystems, Foster City, USA). The RANK, RANKL and OPG gene expression was analyzed by real-time PCR (qPCR) on Applied Biosystems ABI Prism 7000 equipment. Assay on-demand assays designed by Applied Biosystems employing specific oligos and fluorescent Taqman probes were used for each of the PCRs. GAPDH was used to quantify and normalize the expression of the constitutive gene.
In vitro studies:
Primary culture of vascular smooth muscle cells (VSCM)
VSCM from primary culture of aorta explants from healthy Wistar rats at 2 months of age was used, sacrificing 12 rats and using CO2 anesthesia. Abdominal aortas were removed and introduced into cold PBS with 100 units/mL penicillin and 100 mg/mL streptomycin (Biochrom AG, Berlin, Germany). After washing abundantly with cold PBS, the aortas were cut longitudinally; The endothelial layer was carefully removed and subsequently cut into fragments (explants) of 2 to 3 mm2. The explants were plated in six-well culture plates (Sigma-Aldrich) pretreated with fibronectin (10 mg/cm2; Sigma-Aldrich). Once the explants were placed, 1 mL of DMEM (Dulbecco's Modified Eagle Medium, Biochrom AG) supplemented with 20% fetal bovine serum (FBS) (Biochrom AG) was added. The medium was renewed every 2 days. When the cells reached subconfluency, the tissue fragments were removed and the cells were enzymatically separated (0.25% trypsin and 1 mM EDTA).
Cells were seeded at a density of 105 cells per culture dish (Sigma-Aldrich) with DMEM supplemented with FBS (10%). Cells obtained by this method were identified as VSCM by the following criteria: (1) cells grow in the characteristic valley and choline pattern; And (2) immunostaining was positive for alpha-actin (mAb from Sigma-Aldrich).
Cells between passages 2 and 8 were used, using three wells per condition and the experiments were performed in triplicate.
Induction of calcification in VSCM
In order to analyze the uremia-induced calcification and to know the implication of the RANK/RANKL/OPG system, two different conditions were used.
For the first condition, the VSCM cultures were treated with DMEM supplemented with 15% uremic rat serum (a set of 8-week CRF rat sera containing 10.8 mg/dL Ca, 6.7 mg/DL P, and 898 pg/mL PTH). As a control condition DMEM was used with 15% serum from healthy rats (a pool of sera containing 10.4 mg/dL Ca, 3.6 mg/dL P and 25 pg/mL PTH).
In a second condition, to confirm the effect of P, the VSCMs were cultured with calcifying medium: DMEM F12+0.1% bovine serum albumin (BSA) with 2 mM Ca and 3 mM P). DMEM control F12+0.1% BSA was used as condition. In both cases, Ca deposition was determined 4 and 8 days after addition of the stimuli.
The effect of OPG (100 pM), silencing of the RANK receptor (increasing concentrations between 100 pM and 100 nM) was tested in VSCM in which calcification was induced with DMEM F12+0.1% BSA with 2 mM Ca and 3 MM of P.
Gene expression study
We proceeded in the same manner as detailed in the section on in vivo studies.
Lentiviral production and infection/RANK silencing by shRNA
The RANK gene was silenced in the VSCM by small forks of RNA (shRNA), which were cloned into a lentivirus-based vector (FSVsi). In it were introduced shRNAs whose target was TTAGCTGAGGATGCTGAGGAT and scramble sequences. All of them were co-transfected with the virion packaging elements (VDV-G) in a 293T cell culture using polyethyleneimine. Infectious particles were produced by culturing the cells 3-4 days in medium for VSCM. The medium was then centrifuged at 1,000 g for 5 min and the supernatant was added to a VSCM culture, being replaced by the conventional medium after overnight incubation. Finally, the VSCMs were collected after 4 days and the silencing of RANK with qPCR and Western Blot was checked.
Western Blot
After transfer, the membranes were incubated for 12 hours with anti-RANK antibodies (1:1,000, Cells Signaling Technology, Danvers, USA), and anti-tubulin (01:10,000, Sigma-Aldrich). Binding of the secondary antibody was detected with the Western Blot detection kit ECL Advance (Amersham Bioscience, Buckinghamshire, UK) and the VersaDoc 4000 (Bio-Rad) imaging system system.
Statistical analysis
For the statistical analysis of the results, the SPSS 17.0 program was used. In the case of variables with normal distribution, the comparison of the treatment groups was performed using ANOVA with the Bonferroni test. In the case of variables with non-normal distribution, the Kruskal-Wallis test was used.
Results
1. Biochemistry
In the groups that received the diet with a high P content (CRF 8P, CRF 12P, CRF 20P), a slight deterioration of renal function was observed with respect to their controls (CRF 8C, CRF 12C, CRF 20C). Aggravated at 20 weeks (Table 1). In the high P diet group, serum Ca significantly decreased only at week 20 (CRP 20P), while serum P increased in all groups with a high P diet, particularly at 20 weeks of treatment. Parallel to P, PTH increased as treatment time increased, being statistically significant from week 12 and particularly at week 20, where severe secondary hyperparathyroidism was observed (Table 1).
2. Densitometric study
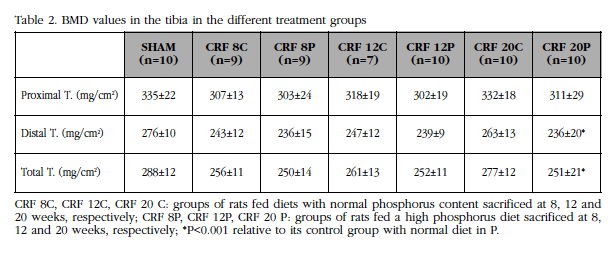
Although there was a slight decrease in the BMD of the groups of animals with high diet in P regarding their controls in all the studied sectors, this was only significant at 20 weeks (Table 2). Losses were predominant at the distal level, where there is a higher content of cortical bone, on losses at the proximal level (Table 2).
3. In vivo effect of uremia and P overload on vascular calcification, bone activity and RANK/RANKL-OPG system
Although the Ca content of the aortas of animals fed a normal P-content diet was slightly affected by uremia, administration of a diet with high P content increased Ca significantly in a time-dependent manner with respect to the Sham group. Animals receiving the high P diet increased the aortic content of Ca with respect to their respective controls from week 12, with this effect being magnified at week 20. Despite the generalized increase in aortic Ca content, von Kossa revealed visible calcifications in the aorta in only 50% of the animals with diet with high content in P (Figure 1).
Parallel to the increase in Ca content there was an elevation of RANKL expression in the aorta (Figure 2A). RANK expression did not show any differences along the course of CRI (Figure 2B), whereas OPG decreased in all uremic groups, particularly those receiving a high P diet (Figure 2C).
In the tibia, an increase in RANKL and OPG expression was observed at week 20 of the high P diet group (Figures 3A, 3C). OPG expression also increased in all groups receiving high P diet, noting the increase observed at week 20. In contrast, RANK expression remained similar in all groups.
4. In vitro effect of uremia and P overload on vascular calcification and the RANK-RANKL-OPG system
Uremic serum induced a significant increase in Ca content at 4 and 8 days (Figure 4A). There was a significant increase in the expression of RANKL (at 4 and 8 days) and OPG (at 8 days of treatment) (Figures 4B, 4C and 4D).
Calcifying medium-treated VMCV (DMEM F12, 2 mM Ca, 3 mM P) showed a significant increase in time-dependent Ca content (Figure 5A). In parallel increased RANKL and OPG expression (Figures 5B, 5C and 5D).
5. In vitro effect of the addition of OPG on calcification induced by uremic serum
To confirm the idea that increased RANKL expression is responsible for the Ca content increase in VSCM treated with uremic serum, 100 pM OPG added to the culture medium, which led to a significant decrease of OPG (Figure 6).
6. In vitro effect of RANK silencing on calcification induced by uremic serum
Similarly, silencing of the RANK receptor by the shRNA technique significantly reduced the Ca content of the VSCM treated with uremic serum (Figure 7).
Discussion
CKD, a disease characterized by a progressive loss of renal function, leads to the appearance of multiple complications and alterations of the cardiovascular system. In order to simulate CKD, we used the animal model with normal CKD from our laboratory.
According to what was observed in the biochemical markers analyzed, the development of CKD was accompanied by alterations in bone and mineral metabolism which were aggravated by hyper-phosphoremia and the development of secondary hyperparathyroidism. It is well recognized that the latter increases bone turnover, negatively affecting the cortical bone (seven/eighths of the tibia or distal area) more severely than the trabecular bone (octave of the tibia or proximal area), which is corroborated in our animals. Although PTH is able to stimulate the expression of OPG, as demonstrated in the tibia of animals with CRF with severe hyperphosphoremia, this hormone is also capable of inducing the expression of RANKL in osteoblasts11, and this gene may be responsible for the BMD decrease recorded in the densitometric study.
The RANK/RANKL/OPG system has also been associated with vascular calcification. Initial evidence of its implication in this process derives from the study with null mice for OPG that, in addition to a severe decrease in BMD and a high incidence of fractures, calcifications of the aorta and renal arteries12.
OPG has the ability to inhibit osteoclastic activity and thus prevent the onset of vascular calcification. In fact, in our in vitro model, vascular calcification induced by uremic serum was attenuated by the addition of OPG.
As some authors have described, a direct relationship between calcification and increase of RANK/RANKL/OPG at the bone level has been observed. The decrease of OPG in aortic tissue induced in our animal model by uremia is in line with what has been reported by other authors. While RANKL was clearly detectable in patients with calcified aortic stenosis, OPG levels were not detectable13. These reductions of aortic OPG by uremia could be due to the process of inflammation during the calcification that occurs with decreases in OPG14,15.
Some authors have described vascular calcification as an active process and regulated by various factors. The VSMC in the early stages of the calcification process undergo a change in its phenotype and begin to express osteogenic markers, which would allow the mineralization of the extracellular matrix. One of these proteins is RANKL, whose expression is abundant in osteoblasts. Both in the aortas and tibias of the rats and in the VSMC there was an increase in the expression of RANKL, aggravated by the increase of P in the diet. In our paper, we show the direct relationship between the increase in calcification and the increase of RANKL. Osteoblasts secrete RANKL, a process that can be reversed by OPG, a protein that sequesters RANKL thus inhibiting the formation of osteoclasts by preventing RANKL from binding to its RANK receptor. The silencing of RANK in our in vitro model of calcification with uremic serum inhibited the calcification process by preventing the binding of RANKL to RANK.
Other studies have also shown that RANKL expression increases in calcified areas16-18, as occurred in the aortas of the animals studied. While in bone an increase of RANKL favors demineralization by an increase in osteoclastic activity, in the vessels it stimulates osteogenesis and, therefore, calcification19. In fact, Kindle L et al. suggest that the endothelial cells of the vessel produce a microenvironment favorable to the formation of calcified tissue, stimulating the migration and adhesion of monocytes through the endothelium that can be differentiated into osteoclasts in the presence of RANKL20. It has recently been demonstrated that VSCM incubated in a calcifying medium to which RANKL is added increases its Ca content and alkaline phosphatase activity, whereas coincubation with OPG is able to inhibit calcification induced by RANKL8.
The hypothesis that the RANK/RANKL/OPG system could explain part of the relationship between osteoporosis and vascular calcification is based on multiple epidemiological studies that have revealed the association between bone and vascular metabolism, noting that the decrease in bone mass and Increased fractures were associated with a higher prevalence and progression of vascular calcifications in the general population and in populations at risk21-26, with the latter being those with CKD.
The Wnt pathway is an intracellular signaling pathway involved in bone formation. Due to the similarities between bone formation and calcification, it has been suggested that the inactivation of the Wnt pathway could attenuate the calcification process, as has been described by several authors27-29. Data from our group, in the same experimental model, have shown an increase in the gene expression of inhibitors of the Wnt pathway in the group of animals with vascular calcification, suggesting a protective mechanism of the progression of calcification30. On the other hand, we should not forget that a negative balance of inhibitors of calcification, such as fetuin A, could also favor the calcification process31,32.
Our in vivo results indicate the involvement of the RANK/RANKL/OPG axis in vascular calcification and changes in BMD as a consequence of CKD and of stimuli favoring the former. Moreover, in our in vitro model, the addition of OPG as well as the silencing of RANK reduced calcification, indicating that the RANK/RANKL/OPG system acts in this process, opening the doors to new investigations in this area. Because of their importance in the regulation of bone turnover, RANK/RANKL/OPG axis members could be used in the future as useful biomarkers in assessing bone function in patients with CKD.
Conflict of interest
The authors declare no conflicts of interest.
![]() Correspondence
Correspondence
Manuel Naves Díaz
Servicio de Metabolismo Óseo y Mineral
Hospital Universitario Central de Asturias
Edificio FINBA, Planta primera F1.1 (Aula 14)
Avenida de Roma, s/n
33011 Oviedo (España)
E-mail: manuel@hca.es
Date of receipt: 07/10/2016
Date of acceptance: 18/10/2016
Bibliography
1. Jono S, Shioi A, Ikari Y, Nishizawa Y. Vascular calcification in chronic kidney disease. J Bone Miner Metab. 2006;24:176-81. [ Links ]
2. Bostrom K, Watson KE, Horn S, Wortham C, Herman IM, Demer LL. Bone morphogenetic protein expression in human atherosclerotic lesions. J Clin Invest. 1993;91:1800-9. [ Links ]
3. Giachelli CM, Bae N, Almeida M, Denhardt DT, Alpers CE, Schwartz SM. Osteopontin is elevated during neointima formation in rat arteries and is a novel component of human atherosclerotic plaques. J Clin Invest. 1993;92:1686-96. [ Links ]
4. Shanahan CM, Cary NR, Metcalfe JC, Weissberg PL. High expression of genes for calcification-regulating proteins in human atherosclerotic plaques. J Clin Invest. 1994;93:2393-402. [ Links ]
5. Boyce BF, Xing L. Biology of RANK, RANKL, and osteoprotegerin. Arthritis Res Ther. 2007;9(Suppl.1):S1. [ Links ]
6. Kong YY, Feige U, Sarosi I, Bolon B, Tafuri A, Morony S, et al. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999;402:304-9. [ Links ]
7. Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165-76. [ Links ]
8. Panizo S, Cardus A, Encinas M, Parisi E, Valcheva P, López-Ongil S, et al. RANKL increases vascular smooth muscle cell calcification through a RANK-BMP4-dependent pathway. Circ Res. 2009;104:1041-8. [ Links ]
9. Hayashi K, Nakamura S, Nishida W, Sobue K. Bone morphogenetic protein-induced MSX1 and MSX2 inhibit myocardin-dependent smooth muscle gene transcription. Mol Cell Biol. 2006;26:9456-70. [ Links ]
10. Mikhaylova L, Malmquist J, Nurminskaya M. Regulation of in vitro vascular calcification by BMP4, VEGF and Wnt3a. Calcif Tissue Int. 2007;81:372-81. [ Links ]
11. Heckt T, Keller J, Peters S, Streichert T, Chalaris A, Rose-John S, et al. Parathyroid hormone induced expression and proteolytic processing of Rankl in primary murine osteoblasts. Bone. 2016;92:85-93. [ Links ]
12. Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Capparelli C, et al. Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998;12:1260-8. [ Links ]
13. Kaden JJ, Bickelhaupt S, Grobholz R, Haase KK, Sarikoç A, Kiliç R, et al. Receptor activator of nuclear factor kappaB ligand and osteoprotegerin regulate aortic valve calcification. J Mol Cell Cardiol. 2004;36:57-66. [ Links ]
14. Crotti T, Smith MD, Hirsch R, Soukoulis S, Weedon H, Capone M, et al. Receptor activator NF kappaB ligand (RANKL) and osteoprotegerin (OPG) protein expression in periodontitis. J Periodontal Res. 2003;38:380-7. [ Links ]
15. Haynes DR, Barg E, Crotti TN, Holding C, Weedon H, Atkins GJ, et al. Osteoprotegerin expression in synovial tissue from patients with rheumatoid arthritis, spondyloarthropathies and osteoarthritis and normal controls. Rheumatology (Oxford). 2003;42:123-34. [ Links ]
16. Min H, Morony S, Sarosi I, Dunstan CR, Capparelli C, Scully S, et al. Osteoprotegerin reverses osteoporosis by inhibiting endosteal osteoclasts and prevents vascular calcification by blocking a process resembling osteoclastogenesis. J Exp Med. 2000;192:463-74. [ Links ]
17. Dhore CR, Cleutjens JP, Lutgens E, Cleutjens KB, Geusens PP, Kitslaar PJ, et al. Differential expression of bone matrix regulatory proteins in human atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2001;21:1998-2003. [ Links ]
18. Jono S, Nishizawa Y, Shioi A, Morii H. 1,25-Dihydroxyvitamin D3 increases in vitro vascular calcification by modulating secretion of endogenous parathyroid hormone-related peptide. Circulation. 1998;98:1302-6. [ Links ]
19. Collin-Osdoby P. Regulation of vascular calcification by osteoclast regulatory factors RANKL and osteoprotegerin. Circ Res. 2004;95:1046-57. [ Links ]
20. Kindle L, Rothe L, Kriss M, Osdoby P, Collin-Osdoby P. Human microvascular endothelial cell activation by IL-1 and TNF-alpha stimulates the adhesion and transendothelial migration of circulating human CD14+ monocytes that develop with RANKL into functional osteoclasts. J Bone Miner Res. 2006;21:193-206. [ Links ]
21. Naves M, Rodriguez-Garcia M, Diaz-Lopez JB, Gomez-Alonso C, Cannata-Andia JB. Progression of vascular calcifications is associated with greater bone loss and increased bone fractures. Osteoporos Int. 2008;19:1161-6. [ Links ]
22. Hak AE, Pols HA, van Hemert AM, Hofman A, Witteman JC. Progression of aortic calcification is associated with metacarpal bone loss during menopause: a population-based longitudinal study. Arterioscler Thromb Vasc Biol. 2000;20:1926-31. [ Links ]
23. Kado DM, Browner WS, Blackwell T, Gore R, Cummings SR. Rate of bone loss is associated with mortality in older women: a prospective study. J Bone Miner Res. 2000;15:1974-80. [ Links ]
24. Boukhris R, Becker KL. Calcification of the aorta and osteoporosis. A roentgenographic study. JAMA. 1972;219:1307-11. [ Links ]
25. Schulz E, Arfai K, Liu X, Sayre J, Gilsanz V. Aortic calcification and the risk of osteoporosis and fractures. J Clin Endocrinol Metab. 2004;89:4246-53. [ Links ]
26. Rodriguez-Garcia M, Gomez-Alonso C, Naves-Diaz M, Diaz-Lopez JB, Diaz-Corte C, Cannata-Andia JB. Vascular calcifications, vertebral fractures and mortality in haemodialysis patients. Nephrol Dial Transplant. 2009;24:239-46. [ Links ]
27. Shalhoub V, Shatzen E, Henley C, Boedigheimer M, McNinch J, Manoukian R, et al. Calcification inhibitors and Wnt signaling proteins are implicated in bovine artery smooth muscle cell calcification in the presence of phosphate and vitamin D sterols. Calcif Tissue Int. 2006;79:431-42. [ Links ]
28. Woldt E, Terrand J, Mlih M, Matz RL, Bruban V, Coudane F, et al. The nuclear hormone receptor PPARgamma counteracts vascular calcification by inhibiting Wnt5a signalling in vascular smooth muscle cells. Nat Commun. 2012;3:1077. [ Links ]
29. Deng D, Diao Z, Han X, Liu W. Secreted frizzled-related protein 5 attenuates high phosphate-induced calcification in vascular smooth muscle cells by inhibiting the wnt/ss-catenin pathway. Calcif Tissue Int. 2016;99:66-75. [ Links ]
30. Roman-Garcia P, Carrillo-Lopez N, Fernandez-Martin JL, Naves-Diaz M, Ruiz-Torres MP, Cannata-Andia JB. High phosphorus diet induces vascular calcification, a related decrease in bone mass and changes in the aortic gene expression. Bone. 2010;46:121-8. [ Links ]
31. Westenfeld R, Schafer C, Smeets R, Brandenburg VM, Floege J, Ketteler M, et al. Fetuin-A (AHSG) prevents extraosseous calcification induced by uraemia and phosphate challenge in mice. Nephrol Dial Transplant. 2007;22:1537-46. [ Links ]
32. Rattazzi M, Bertacco E, Del Vecchio A, Puato M, Faggin E, Pauletto P. Aortic valve calcification in chronic kidney disease. Nephrol Dial Transplant. 2013;28:2968-76. [ Links ]











 text in
text in