Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista de Osteoporosis y Metabolismo Mineral
versão On-line ISSN 2173-2345versão impressa ISSN 1889-836X
Rev Osteoporos Metab Miner vol.9 no.2 Madrid Abr./Jun. 2017
https://dx.doi.org/10.4321/s1889-836x2017000200005
Originales
Screening and biochemical characterization of primary hyperparathyroidism in Guayaquil (Ecuador)
1Servicio Endocrinología - Hospital Docente Policía Nacional Guayaquil Nº 2 - Guayaquil (Ecuador)
2Servicio Endocrinología - Hospital Luis Vernaza - Guayaquil (Ecuador)
Introduction
Primary hyperparathyroidism (HPTP) is a relatively common endocrine disorder. Among endocrine diseases, it ranks third in frequency of diagnosis [1]. HPTP is usually diagnosed in the sixth decade of life and is more common in women [1]. Its clinical presentation has changed in recent decades, evolving from a classical form with significant bone and renal involvement [2], to the asymptomatic form that we are currently seeing [3].
The epidemiology of HPTP has been difficult to establish, since the international literature contains different figures on incidence and prevalence in different populations.
The prevalence of HPTP depends on the populations studied and the detection methods used. In studies of Caucasian populations, it ranges from 1 to 7 per 1,000 adults [4,5,6]. A biochemical screening study has established a prevalence of 1 to 21 per 1,000 adults [7,8,9]. Incidence also varies according to the sources. Incidence studies with PTH and total calcium determinations have been described, in which both high and low rates are reported [5,7,9,10,11,12,13,14,15].
In Latin America, there are few studies on the epidemiology of the disease, with the exception of Eufrazino et al. [16] in Recife (Brazil), Mautalen et al. in Argentina [17], and in Chile by López et al. [18] In Ecuador, PTH prevalence of 7.1% was found in a selected sample of postmenopausal women with low bone mass [19].
The present study would be the first of its kind to evaluate HPTP prevalence in the city of Guayaquil (Ecuador), applying a uniform biochemical screening, by means of the simultaneous measurement of parathyroid hormone (PTH) and serum ionic calcium, and compare our results with those reported in the literature.
Material and methods
This descriptive, prospective and cross-sectional epidemiological study aims to determine the prevalence of PTH during the period between January 1, 2009 and November 30, 2014 in two reference centers in the city of Guayaquil. According to data from the last Population and Housing Census of 2010, provided by the National Institute of Statistics and Censuses of Ecuador (INEC) [20], the population of Guayaquil grew from 2,440,553 to 2,560,505 inhabitants from 2010 to 2014 (Table 1).
Table 1 Projection of the Ecuadorian population by calendar years, according to cantons

tfn3Source: Population and housing census of the year 2010. National Institute of Statistics and Censuses of Ecuador (INEC) [21].
The study was approved by the Ethics and Research Committee at the Guayaquil National Police Teaching Hospital Nº 2.
The diagnosis of PTHP was defined when PTH levels >72 pg/ml (normal values: 12-72 pg/ml) and/or ionic calcium >5.6 mg/dl (normal values: 4.5-5,6 mg/dl) remained elevated on at least two or more different occasions. Serum creatinine, total 25 (OH) vitamin D (D2+D3), and a basic biochemical study (complete blood count, glycemia, liver enzymes, serum lipids, and nitrogen products) were also measured in serum.
Biochemical screening with serum PTH and ionic calcium measurement was carried out in 13,860 people living in the city of Guayaquil (Figure 1), who underwent routine check-ups at the hospitals participating in the study. Patients were treated in primary care units, where they underwent screening tests. Those who returned for routine monitoring and presented serum PTH and/or calcium levels higher than the reference ranges were required to perform an additional assessment of PTH and serum calcium levels.

Figure 1 Design of PHPT screening in Guayaquil (Ecuador)HPTP: primary hyperparathyroidism; PTH: parathyroid hormone; VD: vitamin D.
Serum ionic calcium was measured after 12 hours of fasting and without tourniquet use, under anaerobic conditions (taking the sample in a vacuum tube and uncovering the tube just before the test), and was reported without correction for pH, by direct measurement with selective ion electrode (NOVA-8 equipment), with reference values of 4.5 to 5.6 mg/dl.
Serum PTH (intact molecule) was measured with SIEMENS Immulite 2000 equipment (enzyme-labeled, two-site solid-phase chemiluminescent immunometric assay), with reference values ranging from 12 to 72 pg/ml. The intra-assay precision presented a coefficient of variation of 5.7, 4.3 and 4.2% for concentrations of 72, 258 and 662 pg/ml, respectively, and an interassay coefficient of variation of 6.3 and 8.8% For concentrations of 54 and 387 pg/ml, respectively. The limit of detection was 3.0 pg/ml and linearity up to 500,000 without Hook effect. PTH levels were considered inadequately "normal" when they were above the 75th percentile of the reference value (PTH ≤57 pg/ml) in the presence of hypercalcemia on 2 different occasions.
Serum level of 25 (OH) total vitamin D (total VD=D3+D2) was measured by chemiluminescence, with normal values: 30-70 ng/ml (Centauro kit; competitive 1-step assay with anti-Flurocein). Total precision presented a coefficient of variation of 11.1, 9.6, 9.8, 8.2, 7.8, 4.8% for concentrations of 11.7, 18.0, 32.4, 49, 9, 55.8, 132.1 ng/ml, respectively, with detection limit of 3.2 and linearity up to 150 ng/ml. values ≥30 ng/ml were considered sufficient; mild insufficiency, between 20-29 ng/ml; moderate insufficiency, between 10-19 ng/ml; and severe deficiency, <10 ng/ml [21,22].
Renal function integrity was documented in all cases by measuring serum creatinine levels and calculating endogenous creatinine clearance expressed in ml/min (formula corrected for age, sex, weight and serum creatinine:
[140 – age (years) x weight (kg)] / [72 x creatinine Serum (mg/dl)] x 0.85 (correction factor alone in women).
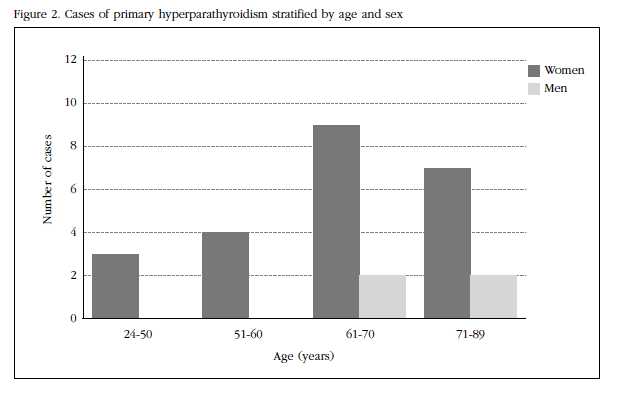
Cases with a high level of calcium and/or PTH on one occasion were considered spurious and excluded. Cases with raised serum creatinine, malabsorption, chronic liver disease, vitamin D insufficiency or those receiving treatment that could alter phosphocalcic metabolism and/or PTH levels (glucocorticoids, estrogens, bisphosphonates, thiazides, lithium , calcium). All biochemical measurements were carried out in a single reference laboratory. All women and men aged 20 years or older were included in the screening sample. Ages ranged from 20 to 89 years. We stratified the cases by sex and age groups: 24-50, 51-60, 61-70 and 71-89 (Figure 2). Data are expressed as mean and standard deviations with the corresponding confidence intervals, 95% confidence level. For the comparison of groups, we used Student's t-test for independent means. A value of p<0.05 was considered statistically significant. Prevalence of the disease was calculated as the number of existing cases divided by the population screened and expressed as a proportion of each population of 1,000 adults. Epidat 3.1 software was used to analyze the data.
Results
Of the 13,860 people evaluated in the screening period, HPTP was found in 27 cases. Patients (n=13,378) with elevated serum creatinine and/or receiving treatments affecting phospocalcic metabolism (estrogens, bisphosphonates, lithium, calcium, thiazides) were excluded. Patients with normal and untreated serum creatinine but who had normal levels of ionic calcium and serum PTH were also excluded. In 482 cases PTH was elevated on at least one occasion of several successive ones; 61 cases had elevated PTH on two or more occasions, but 34 of them had total VD values in the range of insufficiency and were excluded. In the remaining 27 cases, the biochemical diagnosis of PTH was confirmed by raised PTH on two or more occasions (95% CI: 105.01-124.18), preserved renal function (95% CI: 0.765-0.915), and sufficiency of total RV (≥30 ng/ml) (95% CI: 39.12-55.09). In 25 cases (93%), the ionic calcium was in normal ranges (95% CI: 5.0-5.29) and only 2 (women) had minimally elevated values (5.89 and 5.95 mg/dl, respectively).
HPTP was diagnosed more frequently in women than in men (4 men and 23 women), with a 6:1 ratio; the majority of women (87%) were menopausal (n=20).
Table 2 presents the results of the biochemical variables of all patients with confirmed diagnosis of PTHP.
Table 2 Biochemical characteristics of cases with diagnosis of confirmed primary hyperparathyroidism

tfn4PTH: parathyroid hormone. Values are expressed as mean ± standard deviation.
In our series, PTHP was diagnosed more frequently from the sixth decade of life in women, with a mean age of cases around 65 years (95% CI: 57.82-71.12; Range between 24 and 88) and a little later in men, at age 71 (95% CI: 50.95-91.55).
PTH levels far exceeded the upper limit of normal and were similar between women and men (p=ns). In spite of the large increase in PTH levels, in almost all cases serum calcium levels were in the normal range or were slightly higher (range 4.52 to 5.95), and were not different between Men and women (p=ns). Serum levels of total vitamin D were found in the normal range (>30 ng/ml) in 27 cases, and were not different between males and females (p=ns).
In 34 cases vitamin D was in the insufficiency range 21.62±4.7 ng/ml (95% CI: 19.98-23.26) and were excluded from the analysis. In these cases, vitamin D replacement was not performed.
Serum creatinine was within the normal ranges in all cases (95% CI: 0.76-0.91), as well as the endogenous creatinine clearance calculated by the corrected formula.
HPTP prevalence in this sample of the population of Guayaquil corresponds to 2 cases per thousand adults (95% CI: 1.714-2.182). The highest increase in the prevalence of PPH was seen in women ≥60 years and in men ≥70 years (Figure 2).
Discussion
Prevalence studies of HPTP have been carried out mostly in Caucasian populations [4,6,7,10], so that there is no exhaustive information available in other ethnicities and races of our Latin America. Only recently, Yeh MW et al. reported an age-adjusted prevalence of 169.4 and 54.8 per 100,000 women and men in a sub-group of Hispanic race, respectively [11]. The population of Ecuador is multiethnic and the mestizo group is the majority, with an estimated 72% of the total population [20].
Data from epidemiological studies show that, in certain populations at risk, for example, in postmenopausal women and with decreased bone mass, the prevalence ranges from 2.1 to 11.5% [19,23, 24,25].
HPTP is much more common among women, with a ratio of women to men over the age of 60 in the range of 5 to 7:1 [1,17], which is in agreement with our results.
PTHP is recognized as the most common cause of hypercalcemia in outpatient care [26], and in its classic form it has raised levels of PTH, renal lithiasis, and severe bone involvement [1,9,27]. This classic form is still frequently found in developing countries, probably due to the time of delay in diagnosis and the lack of accessibility to measurements of calciotropic hormones and ionic calcium [27].
Another form of presentation of PTHP was identified formally in 2008, identified as normocalcemic HPTP [28], but its description is still incomplete, particularly with respect to its epidemiology, natural history and treatment. Patients with this condition lack the classic HPTP characteristics, and have high levels of PTH with normal serum calcium, which are considered an early sign of the disease [29,30]. The diagnosis should focus on the exclusion of all causes of secondary hyperparathyroidism, particularly vitamin D deficiency (<30 ng/ml) and decreased renal function (endogenous creatinine clearance <60 ml/min) [30].
Normocalcemic HPTP prevalence varies from 0.7 to 16.7% [31,32,33] according to the design of the studies, populations studied, age, sex and methods used. In our series, most cases (93%) had high levels of PTH with ionic calcium in the range of normal or minimally elevated. This would show that the detection was carried out in the early stages of the disease [34] and/or that the predominant form of HPTP presentation in our population is normocalcemic.
The detection of HPTP cases in epidemiological studies has been carried out using a combination of biochemical, histopathological, radiological and clinical data sources [5,6,13,14,35]. However, it should be noted that all data sources have a considerable bias in the results. Taking this into account, our study reveals some findings that are worth highlighting.
Although some controversy persists regarding the usefulness of serum ionic calcium determination, this is a method that reliably allows the diagnostic approach in HPTP. The total calcium concentration does not reliably reflect the predicted increase in the free fraction, especially in cases with minimal or no increase in the total serum calcium level [29,36,37].
Population screening with the simultaneous measurement of serum PTH and ionic calcium at least twice allows us to effectively and safely identify cases. Measuring vitamin D levels and assessing the integrity of renal function allows us to separate the secondary causes of parathyroid hyperfunction. In general terms, the use of our biochemical screening of HPTP would solve the possible research bias obtained in the results of other studies. For example, if only histopathological data were used, there would be a higher detection rate for the minority of patients who are treated surgically. Another bias may also be found in patients with thyroidectomies, where parathyroid adenomas may be found coincidentally in normocalcemic individuals, but these patients cannot be considered cases of PTH [38]. As for radiological studies, they are not an appropriate method for HPTP screening because of their reduced sensitivity and specificity [39].
Among the weaknesses of our study, we pointed out that we did not measure calcium in urine, so we recognize that in our series, the presence of cases with idiopathic hypercalciuria or familial hypocalciuric hypercalcemia cannot be ruled out, although the latter is a rare disease with an estimated prevalence in 1 out of 78,000 people [40]. We also note the inherent limitations of the formula used to calculate endogenous creatinine clearance.
In conclusion, we have characterized the largest series of patients with PHTP described to date in our country and documented the prevalence of HPTP for the first time in our population. Compared to the international series, the prevalence of HPTP is low in this sample and is higher in women and in advanced ages. The biochemical presentation corresponds mostly to the normocalcemic form of the disease.
Our data may help health authorities develop effective strategies for prevention and treatment of skeletal (and non-skeletal) complications of HPTP in our population.
Referencias
1 Fraser WD. Hyperparathyroidism. Lancet. 2009;374 (9684):145-58. [ Links ]
2 Díaz Curiel M, Rapado A, López Gavilanez E, Peramo B, Díez A. Type of bone loss involved in the follow up of surgical or medical treatment of primary hyperparathyroidism. In: Christiansen C, Overgaard K, eds. Proceedings of the third International Symposium on Osteoporosis, vol. 3;1990; Denmark. Copenhagen: Osteopress; 1990.1560-2 p. [ Links ]
3 Bilezikian JP, Potts JT Jr. Asymptomatic primary hyperparathyroidism: new issues and new questions–bridging the past with the future. J Bone Miner Res. 2002;17(suppl 2):N57-67. [ Links ]
4 Christensson T, Hellström K, Wengle B, Alveryd A, Wikland B. Prevalence of hypercalcaemia in a health screening in Stockholm. Acta Med Scand. 1976;200:131-7. [ Links ]
5 Wermers RA, Khosla S, Atkinson EJ, Hodgson SF, O’Fallon WM, Melton LJ III. The rise and fall of primary hyperparathyroidism:a population-based study in Rochester, Minnesota, 1965-1992. Ann Intern Med. 1997;126:433-40. [ Links ]
6 Adami S, Marcocci C, Gatti D. Epidemiology of primary hyperparathyroidism in Europe. J Bone Miner Res. 2002;17(Suppl 2):N18-23. [ Links ]
7 Jorde R, Bønaa KH, Sundsfjord J. Primary hyperparathyroidism detected in a health screening: The Tromsø Study. J Clin Epidemiol. 2000;53(11):1164-9. [ Links ]
8 Stenstrom G, Heedman PA. Clinical findings in patients with hypercalcaemia. A final investigation based on biochemical screening. Acta Med Scand. 1974;195:473-7. [ Links ]
9 Mundy GR, Cove DH, Fisken R. Primary hyperparathyroidism: changes in the pattern of clinical presentation. Lancet. 1980;1:1317-20. [ Links ]
10 Melton III L.J. The Epidemiology of Primary Hyperparathyroidism in North America. J. Bone and Mineral Research. 2002;17(Suppl 2):N12-7. [ Links ]
11 Yeh MW, Ituarte PH, Zhou HC, Nishimoto S, Liu IL, Harari A, et al. Incidence and Prevalence of Primary Hyperparathyroidism in a Racially Mixed Population. J Clin Endocrinol Metab. 2013;98(3):1122-9. [ Links ]
12 Wermers RA, Khosla S, Atkinson EJ, Achenbach SJ, Oberg AL, Grant CS, et al. Incidence of primary hyperparathyroidism in Rochester, Minnesota, 1993–2001: An update on the changing epidemiology of the disease. J Bone Miner Res. 2006;21(1):171-7. [ Links ]
13 Muñoz Torres M, Jodar Gimeno E, Reyes Garcia R, Martínez Díaz Guerra G, Amado JA, Gaztambide S. et al. Results from a national survey on the management of primary hyperparathyroidism. J Endocrinol Invest. 2012;35:957-63. [ Links ]
14 Dedov II, Mokrysheva NG, Mirnaia SS, Rostomian LG, Pigarova EA, Rozhinskaia LI. Epidemiology of primary hyperparathyroidism in Russia (the first results from the database of federal state institution ‘Endocrinological Research Centre’. Problemy Endokrinologii. 2011; 57(3):3-10. [ Links ]
15 Griebeler ML, Kearns AE, Ryu E, Hathcock MA, Melton LJ 3rd, Wermers RA. Secular trends in the incidence of primary hyperparathyroidism over five decades (1965-2010). Bone. 2015;73:1-7. [ Links ]
16 Eufrazino C, Veras A, Bandeira F. Epidemiology of primary hyperparathyroidism and its nonclassical manifestations in the city of Recife, Brazil. Clin Med Insights Endocrinol Diabetes. 2013;6:69-74. [ Links ]
17 Mautalen CA, Gallo Morando C, Torres Agüero M, Barcat JA, Arata RO, Molins M. Tratamiento quirúrgico del hiperparatiroidismo. Medicina (B Aires). 1972;32:150-8. [ Links ]
18 López JM, Sapunar J, Campusano C, Arteaga E, Rodríguez JA, León A, et al. Changes in the clinical presentation of primary hyperparathyroidism. Analysis of 84 cases. Rev Med Chil. 1993;121(3):265-72. [ Links ]
19 López Gavilanez E, Huamán Garaycoa F, Segale Bajaña A, Castillo Delvalle M, Macías Briones G. Screening for primary hyperparathyroidism in women with low bone mass. Osteoporosis Int. 2006;17(Suppl 2):S191. [ Links ]
20 http://www.ecuadorencifras.gob.ec/censo-de-poblacion-y-vivienda (accedido el 1 diciembre 2015). [ Links ]
21 Mithal A, Wahl DA, Bonjour JP, Burckhardt P, Dawson-Hughes B, Eisman JA, et al. IOF Committee of Scientific Advisors (CSA) Nutrition Working Group. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos Int. 2009;20(11):1807-20. [ Links ]
22 Mori H, Okada Y, Tanaka Y. Incidence of vitamin D deficiency and its relevance to bone metabolism in Japanese postmenopausal women with type 2 diabetes mellitus. Intern Med. 2015;54(13):1599-604. [ Links ]
23 Lundgren E, Hagström EG, Lundin J, Winnerbäck K, Roos J, Ljunghall S, et al. Primary hyperparathyroidism revisited in menopausal women with serum calcium in the upper normal range at population-based screening 8 years ago. World J Surg. 2002;26(8):931-6. [ Links ]
24 Lundgren E, Rastad J, Thrufjell E, Akerström G, Ljunghall S. Population-based screening for primary hyperparathyroidism with serum calcium and parathyroid hormone values in menopausal women. Surgery. 1997;121(3):287-94. [ Links ]
25 Misiorowski W, Zgliczyński W. Prevalence of primary hyperparathyroidism among patients with low bone mass. Adv Med Sci. 2012;57(2):308-13. [ Links ]
26 Lafferty FW. Differential diagnosis of hypercalcemia. J Bone Miner Res. 1991;6(S2):S51-8. [ Links ]
27 González L, López Gavilanez E, Tagle M, Morán B. Síndrome Neuromuscular Severo, hipercalcemia y tumor óseo. Rev Esp Enf Metab Oseas. 1997;6(5)181-4. [ Links ]
28 Bilezikian JP, Khan AA, Potts JT Jr. Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the third international workshop. J Clin Endocrinol Metab. 2009;94(2):335-8. [ Links ]
29 López Gavilanez E, Huaman Garaycoa F, Garcés Santos JC, Marengo Baquerizo C, Reyes Aguirre G. Osteoporosis, elevated PTH and inadequately "Normal" Ionic Calcium. Rev Esp Enf Metab Oseas. 2005;14(3):52-4. [ Links ]
30 Martínez Díaz-Guerra G; Jódar Gimeno E; Reyes García R; Gómez Sáez JM; Muñoz-Torres M. Normocalcemic primary hyperparathyroidism Recommendations for management and follow up 2013. Endocrinol Nutr. 2013;60(8):456.e1-5 [ Links ]
31 Cusano N, Wang P, Cremers S, Haney E, Bauer D, Orwoll E, et al. Asymptomatic normocalcemic primary hyperparathyroidism: characterization of a new phenotype of normocalcemic primary hyperparathyroidism. Poster session presented at:"33rd Annual Meeting of the American Society of Bone and Mineral Research"; September 16-20, 2011.San Diego, California. SU0167. [ Links ]
32 Garcia-Martin A, Reyes-Garcia R, Munoz-Torres M. Normocalcemic primary hyperparathyroidism: one-year follow-up in one hundred postmenopausal women. Endocrine. 2012;42(3):764-5. [ Links ]
33 Berger C, Langsetmo L, Hanley D, et al. Relative prevalence of normocalcemic and hypercalcemic hyperparathyroidism in a community-dwelling cohort. Poster session presented at:"33rd Annual Meeting of the American Society of Bone and Mineral Research"; September 16-20, 2011. San Diego, California. SU0173. [ Links ]
34 Maruani G, Hertig A, Paillard M, Houillier P. Normocalcemic primary hyperparathyroidism: evidence for a generalized target-tissue resistance to parathyroid hormone. J Clin Endocrinol Metab. 2003;88 (10):4641-8. [ Links ]
35 Yu N, Donnan PT, Murphy MJ, Leese GP. Epidemiology of primary hyperparathyroidism in Tayside, Scotland, UK. Clin Endocrinol. 2009;71(4):485-93. [ Links ]
36 Ladeson JH. Calcium Determination in Primary Hyperparathyroidism. J Bone Miner Res. 1991;6(Suppl 2):S33-41. [ Links ]
37 Tee MC, Holmes DT, Wiseman SM. Ionized vs serum calcium in the diagnosis and management of primary hyperparathyroidism: Which is superior?. Am J Surg. 2013;205(5):591-6. [ Links ]
38 Carnaille BM, Pattou FN, Oudar C, Lecomte-Houcke MC, Rocha JE, Proye CA. Parathyroid incidentalomas in normocalcemic patients during thyroid surgery. World J Surg. 1996;20(7):830-4. [ Links ]
39 Udelsman R, Pasieka JL, Sturgeon C, Young JEM, Clark OH. Surgery for asymptomatic primary hyperparathyroidism: proceedings of the Third International Workshop. J Clin Endocrinol Metab. 2009;94(2):366-72. [ Links ]
40 Hinnie J, Bell E, McKillop E, Gallacher S. The prevalence of familial hypocalciuric hypercalcemia (FHH). Calcif Tissue Int. 2001;68(4):216-8. [ Links ]
Received: January 02, 2017; Accepted: March 07, 2017











 texto em
texto em