My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista de Osteoporosis y Metabolismo Mineral
On-line version ISSN 2173-2345Print version ISSN 1889-836X
Rev Osteoporos Metab Miner vol.9 n.2 Madrid Apr./Jun. 2017
https://dx.doi.org/10.4321/s1889-836x2017000200006
Revisión
Thyroid hormones, TSH, thyroid cancer and bones in pre- and postmenopausal women
1Instituto de Investigación Hospital 12 de Octubre (i+12) - Facultad de Medicina - Universidad Complutense de Madrid (España)
Introduction
Thyroid hormones (HT) are involved in skeletal development, peak bone mass acquisition, and maintenance of bone remodeling. Clinical-epidemiological studies indicate that both deficiency and excess of HT are associated with risk of fractures, with euthyroidism being considered as fundamental for the normal functioning of bone remodeling [1].
This "homeostatic" response to HT is regulated at different levels, but in particular by the conversion of thyroxine (T4) to triiodothyronine (T3) by iodothyronine deiodinases, responsible for the latter acting on its peripheral receptors.
In this paper, we will review the cellular actions of HT on bone, and especially the in vivo experimental model of thyroid stimulating hormone excess and suppression (TSH) in patients with differentiated thyroid carcinoma (CDT) in women Pre and postmenopausal. In men with CDT there are no longitudinal quality studies for analysis.
Thyroid hormones and bone
HT and bone are closely related, since HT are key regulators of bone remodeling. HT plays a key role in the growth and development of vertebrates. HT are iodothyronines synthesized in the thyroid gland, whose constant secretion is ensured by two mechanisms: 1) secretion of HT controlled by a retroactive mechanism, hypothalamic-pituitary-thyroid gland axis (Figure 1), and 2) by regulated intracellular activation by iodothyronine-deiodinases [2].
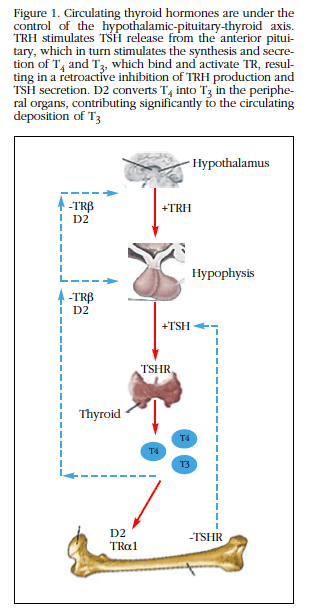
Figure 1 Circulating thyroid hormones are under the control of the hypothalamic-pituitary-thyroid axis.TRH stimulates TSH release from the anterior pituitary, which in turn stimulates the synthesis and secretion of T4 and T3, which bind and activate TR, resulting in a retroactive inhibition of TRH production and TSH secretion. D2 converts T4 into T3 in the peripheral organs, contributing significantly to the circulating deposition of T3
Thyroid stimulating hormone (TSH) produced by the thyrotrophic cells of the pituitary gland, promotes the synthesis and secretion of HT, mainly 3, 5,3',5'-tetraiodothyronine (T4), or thyroxine. It is considered that T4 behaves as a prohormone that needs to be converted to the 3,3',5 triiodothyronine (T3), which is more potent and is considered biologically active, which is carried out through a 5'-monodeiodination Present in tissues. If iodine cleavage is position 5, this molecule results in the inactive metabolite 3,3',5'-triiodothyronine, or reverse T3 (rT3), with weak agonist activity on the same receptors as T3.
The thyroid gland secretes T4 and also small amounts of T3, the active hormone. The majority of circulating T3 originates from the deiodination of T4 in peripheral tissues. To perform genomic action, T4 must be converted to T3 (Figure 2). Of the three deiodinases involved in the metabolism of HT, deiodinase type 1 (D1), which is expressed mainly in the thyroid gland, is the main responsible for the transformation of T4 to T3. It is estimated that D2 intervenes in the control of its concentrations, contributing to limit the access of the HT to the tissues, during the processes of tissue development and repair. The joint action of D2 and D3 would be responsible for the intracellular control of the availability of T3 [3].
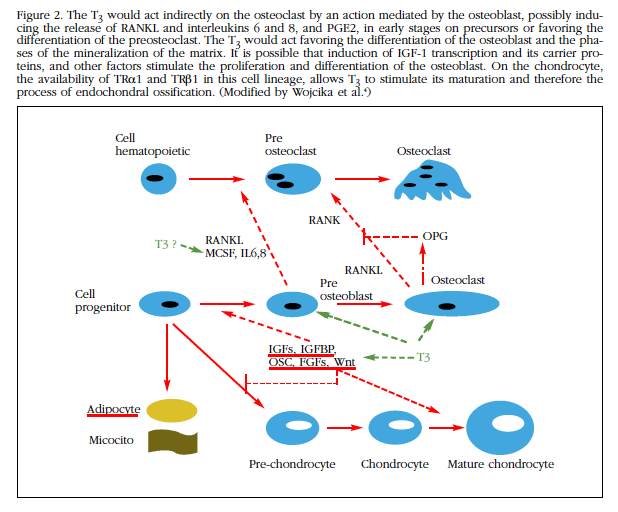
Figure 2 The T3 would act indirectly on the osteoclast by an action mediated by the osteoblast, possibly inducing the release of RANKL and interleukins 6 and 8, and PGE2, in early stages on precursors or favoring the differentiation of the preosteoclast.The T3 would act favoring the differentiation of the osteoblast and the phases of the mineralization of the matrix. It is possible that induction of IGF-1 transcription and its carrier proteins, and other factors stimulate the proliferation and differentiation of the osteoblast. On the chondrocyte, the availability of TRα1 and TRβ1 in this cell lineage, allows T3 to stimulate its maturation and therefore the process of endochondral ossification. (Modified by Wojcika et al.4)
The uptake of thyroid hormones by tissues is carried out by specific transporter proteins. Both T4 and T3 enter the target cells through membrane-specific transporters, including monocarboxylate transporters 8 and 10 (MCT8 and MCT10) and OATP1c1 [4]. The best studied was the MCT8 monocarboxylated transporter, with inactivating mutations in gene 8 located on the X chromosome of this protein that cause Allan-Herndon-Dudley syndrome, with high concentrations of HT and neurological abnormalities, as well as hearing disorders [5].
Receptors for HT
Once in the cellular interior, deiodinase D2 converts T4 to T3 and deiodinase D3 inactivates both T3 and T4, converting them to T2 and T3 reverse. T3 enters the nucleus where 3 types of thyroid hormone (TR) receptors are found: TRα1, TRβ1 and TRβ2, to which it binds by forming a heterodimer with the retinoid X receptor (RXR), which binds in turn to The DNA sequence termed the "HT response element" (TRE) of the T3 target gene, controlling its expression [1].
These three functional receptors for HT (TRα1, TRβ1 and TRβ2) are encoded by the THRα and THRβ genes, which regulate their expression and transcriptional responses to TR. The expression of TRα1 and TRβ1 has been described in the bone, the former being in predominant concentrations of 10: 1. It is considered, therefore, that TRα is the fundamental mediator of T3 action on the bone [6].
HT, TSH and bone development
Eutyroidism is essential for the normal development of the skeleton. This is carried out through the process of intramembranous ossification (differentiation of mesenchymal progenitors into cells forming osteoblasts) and endochondral ossification, through which the long bones form a cartilage mold. Chondrocytes are formed from the mesenchymal precursors to form this cartilage mold; In the primary ossification center of this occurs the progressive mineralization. Vascular invasion and emigration of osteoblasts transform this area into trabecular bone; The precursors located in the most peripheral mesenchyme in the perichondrium are differentiated into osteoblasts and form cortical bone. This proliferation and longitudinal growth continues to maturity [1,2,3].
Both the TRα1 receptor and the TRβ1 are expressed in the chondrocytes of the growth plates, suggesting that they are targets for the action of T3. Chondrocyte proliferation and differentiation is controlled by Indian hedgehog, PTHrp, BMP-R1A, IGF1, Wnt, and FGFs. The first three by a negative feedback that induces plaque growth and inhibits its differentiation by controlling its linear growth. The HT intervene in this regulation, sensitive to the availability of T3, which stimulates gene expression for the synthesis of cartilage matrix and its subsequent mineralization.
In osteoclasts it has not been possible to establish that T3 has effects through the functional receptors expressed in these cells, being possible that they are indirect mediated through the osteoblasts. In states of excess of HT an increase in the number and activity of osteoclasts, as well as bone loss, is detected. T3 also stimulates the differentiation of osteoblasts, the synthesis and mineralization of the bone matrix; These effects are carried out through the regulation of procollagen enzymes, including bone alkaline phosphatase, and metalloproteinases 9 and 13 [7]. It is not yet clear whether these effects are mediated via the activator receptor ligand pathway For nuclear factor κB (RANKL) [8], although studies with cell cultures of osteoblasts or precursors, demonstrate that T3 increases the expression of RANKL and interleukins 6 and 8 [9].
It is possible that the action of T3 on osteoblasts is mediated by the expression of osteoprotegerin, which would act by inhibiting RANKL, which in turn stimulates osteoclastogenesis. What has been demonstrated is that T3 induces the transcription of IGF1, while stimulating its IGF1BP-2 and IGFBP-4 transport proteins, which, together with the increased activity of alkaline phosphatase (and, therefore, Better quality of mineralization) and the other effects already described, behaves as a stimulator of osteoblastic activity at different levels [1]. The TSH-thyroid axis is necessary for this normal skeletal development; TSH has a direct effect on bone, as demonstrated by in vitro studies in which it behaves as a direct inhibitor of bone remodeling, through acting on TSHR expressed in osteoblasts and osteoclasts. In relation to the skeletal development phase, TSH alterations are implicated in three diseases: 1) in congenital and acquired hypothyroidism that can cause decreased bone remodeling and increased risk of fractures; 2) in hyperthyroidism, with actions contrary to the previous one, greater remodeling, but also greater risk of fractures; and 3) in craniosynostosis with premature closure of cranial sutures, osteoporosis and fractures. However, since there are circulating levels of HT in these diseases, their effects can not be separated from the action of TSH on bone; The description that isolated TSH deficiency with a mutation affecting the beta-subunit TSH is characterized by a shortened metacarpal and metatarsal phenotype but with normal bone mineral density (BMD) response after treatment with HT in the absence of TSH has led to suggest that the predominant role on bone development corresponds to T3.
Recently a heterozygous mutation in the THRα gene has been described in a 6-year-old girl, who had HT at the low or normal limit and normal TSH, had growth retardation and histological bone involvement similar to hypothyroidism, which implies a Important role for these TRα receptors in human bone development [10].
In adults, hypothyroidism is characterized by decreased bone remodeling with less osteoclastic resorption and less bone formation. This implies a longer duration of the bone remodeling cycle, with an increase in the secondary period of mineralization. This could lead to an increased risk of fractures in these individuals. In contrast, in adult hyperthyroidism, there is a high bone remodeling with osteoporosis characterized by an increase in net bone resorption. There are also more fractures and lower bone mineral density.
HT and TSH in relation to bone mineral density and fractures in normal population
There are prospective studies in premenopausal and postmenopausal women assessing the effect of TSH and HT levels on BMD in the normal population. Kim et al. Studied the relationship between circulating T3 and TSH and its effect on bone mass in healthy subjects [11]. In a population of 37,431 adults performed BMD measurement and thyroid function test, excluding diseases that may affect these parameters. Low levels of TSH and elevated T3 were associated with lower BMD values at all skeletal sites, and confirmed a protective effect of TSH on bone loss independent of the effect of T3. The negative impact of T3 on BMD could be offset by an increase in TSH only in those with T3 levels in the normal-high range.
Studies in relation to fracture risk and bone loss and TSH levels have been conflicting. TSH levels in the low-normal range were associated with hip fractures in elderly women [12]; While, in the same vein, a study of younger postmenopausal women showed that levels above the normal range were associated with a 35% reduction in the risk of non-vertebral fractures [13]. Finally, a meta-analysis performed with 70,298 participants described a risk of hip fractures of 1.61 (95% CI: 1.21-1.15) and for other fractures of 1.98 (95% CI: 1.41 -2.78) in patients with subclinical hyperthyroidism with TSH levels <0.10 mIU/L [14].
The history of hyperthyroidism appears to be a risk factor. In the SOF (Study of Osteoporotic Fractures) study of 192 elderly women with a follow-up of 4.1 years, the highest incidence of osteoporotic fracture was recorded in patients with a history of fractures and/or hyperthyroidism [15]. In this study, no evidence was found to correlate low TSH levels with low BMD. The authors concluded that hyperthyroidism may or may not reduce bone mass, but that in their study the decline in BMD was not responsible for the strong association between prior hyperthyroidism and the risk of hip fracture.
HT and TSH: relationship with bone mineral density and fractures in women with thyroid dysfunction
Clinical hyperthyroidism is recognized as a risk factor for bone loss, promoting bone turnover and trabecular perforation. In relation to endogenous hyperthyroidism (Graves' disease, multilocular toxic goiter), the data indicate that it may also increase the risk of fractures in general and/or vertebral fractures in postmenopausal women. The prospective study by Bauer et al. [16] showed that hyperthyroid women with TSH levels <0.1 mU/L, compared to euthyroid controls, had a three-fold increased risk of hip fracture (OR: 3.6, CI 95%: 1.0-12.9) and four times of vertebral fracture (OR: 4.5; 95% CI: 1.3-15.6).
In a study by Baqi et al. In premenopausal women receiving oral levothyroxine (LT4), there was a significant correlation between BMD at the lumbar spine (CL) level and hip and TSH levels, as well as a negative correlation between TSH levels and markers Osteocalcin and N-terminal telopeptide of type I collagen (NTX) [17]. The results were more favorable for BMD and levels of bone remodeling markers (MRO) in patients with TSH >0.3 mU/l than those with values <0.3 mU/l.
However, at the level of subclinical hyperthyroidism (TSH suppressed with thyroid hormones in normal range), the effects of HT on bone are more controversial. A prospective study of 2,004 patients with subclinical hyperthyroidism reported a 1.25-fold increase in fracture in these, similar to the 1.9-fold increase in fracture risk found in patients treated with T4 [18,19]. However, a recent study by Garin et al., Conducted in 4,936 subjects over 65 years of age for 12 years, found no relation between the risk of hip fracture and subclinical hyperthyroidism [20].
Two meta-analyzes of postmenopausal studies with subclinical hyperthyroidism due to exogenous substitution have found a decrease in BMD with an annual loss of 0.91% of bone mass [21,22]. The meta-analysis of Wirth et al., Which includes only 5 published studies with a high quality index, concludes that subclinical hyperthyroidism may be associated with a risk of 2.16 (95% CI: 0.87-5.37) For hip fractures and 1.43 (95% CI: 0.73-2.78) for non-vertebral fractures [23].
Most studies in postmenopausal women show an association between high-normal levels of HT and lower BMD values, with an increased risk of non-vertebral fracture. Kim et al. [24] studied the results of BMD in a group of 959 women with subclinical hyperthyroidism (TSH <0.5 mIU/L) vs A group with TSH >0.5 mU/L. Women with TSH values in the normal-low limit maintained lower BMD values in the spine and femoral neck than those with TSH in the normal-high limit. The former also had a 2.2-fold increased risk of osteoporosis. Similarly, Morris et al. [25], in a sample of 581 healthy American women, describe a higher risk of osteoporosis in women with TSH values at the low-normal limit (0.39-1.8 mIU/L) with (OR: 3.4 [95% CI: 1.3-9.2] and 2.2 [95% CI: 1.2-3.8], respectively).
In summary, published data indicate that to demonstrate clear causality, randomized and controlled trials with a large number of patients are necessary, and to assess whether normalization of TSH levels in subclinical hyperthyroidism is associated with fracture risk. The data suggest that subclinical hyperthyroidism is associated with an increased risk of hip and non-vertebral fractures, but other factors should be analyzed and studies of higher quality should be performed.
In clinical hypothyroidism there is a decrease in bone formation that usually exceeds the decrease in resorption, as confirmed by histomorphometry data. In general, the existence of a normal BMD has been described, contrasting with an increase of 2 to 3 times the frequency of fractures, particularly of forearm in some series. In postmenopausal women with subclinical hypothyroidism, a similar risk of fractures has also been reported, especially those with autoimmune origin [26].
HT and bone trabecular microarchitecture
It has been commented on the possibility that the bone quality, determined by the trabecular microstructure, could also be influenced by the thyroid state. In this sense, Basset et al. Have shown thinning and decreased trabecular connectivity in a mouse model with thyrotoxicosis [27].
More recently, Hwangbo et al. Have studied 1,376 euthyroid subjects (648 postmenopausal) in which they determine HT, free T4 and trabecular bone score (TBS) [28]. TBS is the technique by which, based on lumbar DXA scanning, it establishes textural gray levels as indirect indices of microarchitecture. They conclude that elevated levels of free T4 were associated with impairment of trabecular microarchitecture, whereas TSH levels were not associated with lumbar TBS. This would support the results described in mice resistant to HT, in which it has been shown that elevated HT rather than TSH predominate in the regulation of bone state.
Criteria for thyroid suppression in differentiated thyroid cancer
Differentiated thyroid carcinoma (CDT) is the most common endocrine neoplasia (accounting for 1% of all cancers). 85-90% of thyroid cancers are CDT, which includes two variants, the papillary carcinoma (the most frequent) and the follicular carcinoma. Its incidence has increased in the last 10 years, but its mortality rate remains the same [29]. This increase is due in large part to the increase and improvement of resolution of the diagnostic tests, with greater detection of incidental microcarcinoma.
Treatment indicated in the CDT includes total thyroidectomy completed with ablative dose of radioactive iodine. Subsequently, based on the risk of relapse, a dose of oral replacement (very low risk) or suppressive levothyroxine is given. The suppressive dose aims to induce hyperthyroxinemia with pituitary suppression of TSH that could be a potential stimulus for tumor remnants. The initial suppressive dose of levothyroxine is calculated at 1.8-2.2 µg/kg/day, which is modified according to successive controls. Based on the suppression obtained during hormone therapy, the American Thyroid Association (ATA) has established the following risk groups for treatment with levothyroxine: 1) Low-risk group >0.5 mIU/L; 2) Intermediate risk group: 0.1-0.5 mIU/L and; 3) High risk group: <0.1 mIU/L. Patients with exogenous treatment (by CDT) as well as those with endogenous hyperthyroidism are subjected to prolonged periods of the effect of thyroid hormones on the bone. Many of the aspects related to the bone loss that this therapy can cause, either directly or by suppression of the pituitary-thyroid axis, are now known.
Suppression of TSH in thyroid carcinoma. Bone loss and relation to the risk of relapse
Treatment with levothyroxine in CDT is based on doses that suppress serum TSH levels below the normal range, resulting in a condition similar to that of subclinical hyperthyroidism. We have already pointed out how TSH behaves as a stimulus for the proliferation of thyroid cells, in addition to the uptake of radioiodine and the production of thyroglobulin, so suppression seeks to remove this effect and prevent a recurrence. TSH receptors have been described in the membranes of CDT tumor cells whose concentrations are affected by the reduction of TSH by levothyroxine therapy [30]. There are also observational epidemiological studies in which a positive correlation has been found between elevated serum TSH levels and risk of malignancy in nodules or more advanced stages of CDT (Table 1). Finally, McGriff et al., in a meta-analysis involving 4,174 patients with CDT, demonstrated a decreased risk of tumor progression in patients receiving levothyroxine suppressive therapy (RR=0.73; 95% CI: 0.6-0.88, p<0.05) [31].
Table 1 TSH targets for prolonged treatment with thyroid hormone in differentiated thyroid carcinoma. According to Haugen BR et al.32
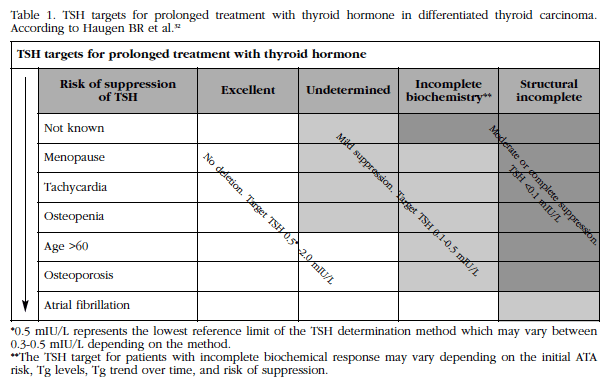
tfn3*0.5 mIU/L represents the lowest reference limit of the TSH determination method which may vary between 0.3-0.5 mIU/L depending on the method. **The TSH target for patients with incomplete biochemical response may vary depending on the initial ATA risk, Tg levels, Tg trend over time, and risk of suppression.
Although there is no general consensus about optimal TSH levels to decrease relapses and minimize the adverse effects of subclinical hyperthyroidism, the American Thyroid Association (ATA) recently defined the impact of TSH suppression in patients with CDT Characterized by low, intermediate and high risk of relapses taking into account several clinical factors [32].
It should be noted that previously Biondi and Cooper, in a review, concluded that aggressive suppression of TSH is important in patients with CDT and high risk, and is much less critical in the other groups [33]. Based on these criteria, Wang et al. Recently studied 306 non-suppressed patients and 465 suppressed patients with CDT classified as low or intermediate risk, and who presented similar recurrence rates after 6 years of follow-up [34]. However, patients with TSH suppression <0.4 mIU/L had a higher incidence of osteoporosis and atrial fibrillation compared with non-suppressed patients (HR 2.1; p=0.05), meaning that prolonged treatment with levothyroxine with Suppressive effect increases the risk of postoperative osteoporosis in patients with low and moderate risk of CDT, according to the ATA classification.
It can be concluded that the optimal dose of maintenance of TSH in patients with CDT of low or intermediate risk of relapse has not yet been well established. Studies suggest that a level of 0.9-1.0 mIU/L could be the optimal suppression value for low- and intermediate-risk CDTs, in order to further reduce the development of osteoporosis and long cardiologic complications Term, without increasing the risk of relapse. It is possible, therefore, that TSH suppression is an independent predictor of bone damage that, moreover, does not seem to diminish relapses in these low- and intermediate-risk patients.
Impact of TSH suppression: adverse effects and quality of life
The prescription of thyroid hormones is ample, reaching almost 5.1% of the adult population. It is generally a well tolerated medication and few immediate side effects. In the last years, publications are being made regarding whether levothyroxine therapy increases the incidence of fracture in the long term. Current evidence is not definitive, although Turner et al. Showed an increase in fractures in elderly patients (>70 years) treated for long periods with thyroxine [35]. The mechanisms by which thyroxine would induce these fractures are unknown, but it has been suggested that bone mineral density would be decreased through induction of subclinical hyperthyroidism, or that normal-high levels cause it . The greater frequency of falls due to arrhythmias favored by this increase of thyroid hormones would be another cause.
The main adverse effects of TSH suppression affect the cardiovascular system, bone metabolism and quality of life (Table 2). In clinical hyperthyroidism, the incidence of atrial fibrillation, myocardial infarction, and mortality increased markedly in the elderly [36]. It is known that atrial fibrillation can triple in the course of 10 years of treatment (TSH <0.1 mIU/L) in those over 65, euthyroid subjects (TSH at the limit of normal). In subclinical CDT hyperthyroidism, in patients treated with levothyroxine, the risk of atrial fibrillation may reach 10.3% (17.5% in the >60 years), according to a study conducted in a population-based population register Million in Denmark [37]. Finally, overall mortality has also been increased (OR: 1.20, 95% CI: 1.06-1.36) in situations of hyperthyroidism in patients with TSH <0.03 mIU/L, compared with those of those With values ranging from 0.04 to 0.4 mIU/L [38].
Table 2 Relationship of longitudinal studies on the effect of TSH suppression with levothyroxine on bone mineral density (BMD) in pre and postmenopausal women with thyroid cancer
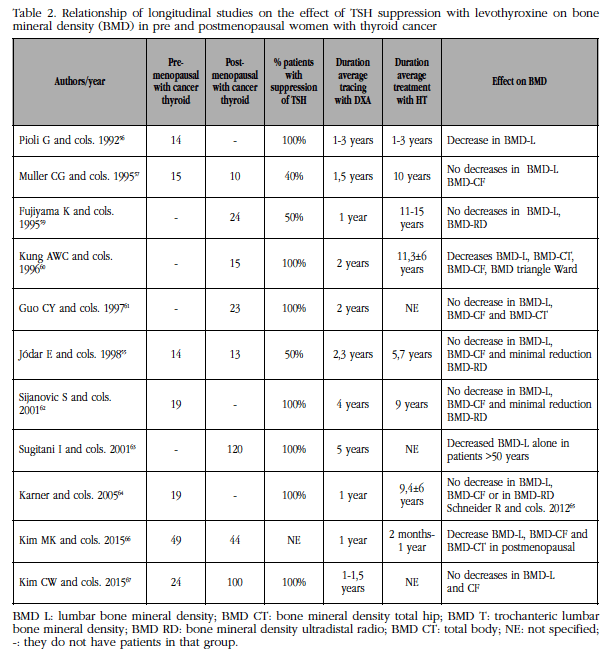
tfn4BMD L: lumbar bone mineral density; BMD CT: bone mineral density total hip; BMD T: trochanteric lumbar bone mineral density; BMD RD: bone mineral density ultradistal radio; BMD CT: total body; NE: not specified; -: they do not have patients in that group.
The increase of thyroid hormones can cause emotional alterations (nervousness, anxiety), mood disorder (depression, sleep disorders, asthenia) and various cognitive alterations, which can influence the quality of life of the patient. Samuel et al. Describe greater items of fatigue and depression in patients treated with levothyroxine suppressive doses [39]. Jarcas et al. Reported cognitive alterations in 31 patients with CDT and suppressive therapy with thyroid hormones [40]. In front of these, Moon et al. Have pointed out that the cognitive functions studied in a group of 50 patients with CDT over 65 years were positively correlated with the higher serum T4 elevation of these patients in relation to the controls [41].
An observational study by Flynn et al. has studied the effects on the cardiovascular system and fractures in a population of 17,684 subjects on prolonged T4 [38] treatment. They found that patients with elevated (>4 mIU/L) or suppressed (<0.03 mIU/L) TSH had an increased risk of cardiovascular disease, with HR=1.95 (95% CI: 1.73-2.21), for arrhythmias of 1.80 (95% CI: 1.33-2.44) and for fractures of 1.83 (95% CI: 1.41-2.37); had low but not suppressed TSH (0.04-0.4 mIU/L) did not present increased risk in any of these objectives. These authors conclude that it might be safe for patients who ingest T4 to maintain low but not suppressed TSH.
Thyroid hormones, thyroid suppression and differentiated thyroid cancer
Clinical hyperthyroidism is a recognized risk factor for bone loss, promoting bone remodeling, trabecular perforation, and increased risk for fractures. At the level of subclinical hyperthyroidism (TSH suppressed with normal range HT) the effects of HT on bone are more controversial. Experimental studies and clinical data have demonstrated that thyroid cell proliferation is dependent TSH [42]. The start of treatment with suppression of TSH causes a situation of subclinical hyperthyroidism. Baliran et al. [43] have shown that excess of HT and low TSH levels stimulate bone resorption. This should be taken into account, given the general good prognosis of these patients, which could lead to the appearance of fractures in prolonged periods of suppressive therapy. In general, the studies describe more aggressive treatments for suppression of TSH in patients at high risk of disease or tumor recurrence, while a less aggressive suppression seems advisable in patients with low risk. In addition, it should be noted that, in recent years, the increase in the prevalence of papillary microcarcinomas with good survival requires modification of these suppression criteria. The maintenance of TSH numbers in the normal range may be advisable for long-term treatment in patients with advanced CDT and relapse-free.
Suppressive treatment with HT in cancer differentiated from thyroid and bone. Longitudinal studies vs transverse
To date, a large number of cross-sectional studies have been published on the effect of suppressive therapy with HT on CDT in both premenopausal and postmenopausal women. In premenopausal studies, there are three studies that find a decrease in BMD in some of the studied areas [44,45,46]. In front of them, there are three times more studies that do not find any deleterious effect of TSH suppression on the bone in these patients [47].
In postmenopausal patients with CDT, there is a greater disparity of results: some report a decrease in lumbar and neck BMD, and in some, there is also bone loss in radio [46,48,49], in contrast to a large majority who register changes in BMD with Suppressive treatment [50,51,52,53]. It is possible that the heterogeneity of the thyroid cancer patients selected for the studies, the different levels of TSH suppression and the different techniques used for hormonal determinations and bone mineral density may influence the significant differences of these results.
For the above reasons, we believe that the study of bone mass follow-up in these patients with CDT is of more value, disregarding cross-sectional studies that reflect a specific situation. Compared with longitudinal studies, cross-sectional analyzes are more susceptible to sample error and other bias [54]. The objective was to review the publications with prospective criteria, the possible bone losses in the different areas studied with bone densitometry, with time of treatment and detailed follow-up, as well as the criteria and times of TSH suppression of these patients. Following these objectives we found in PubMed 11 publications, with longitudinal follow-up, including one from our group [55], which we will analyze next (Table 2).
The first longitudinal study was Pioli et al. [56], who studied 14 premenopausal patients (age 43±6.8 years) with TCD, with densitometries every six months and during follow-up with levothyroxine reaching 3 years. Although ten of these patients underwent almost total thyroidectomy and 5 to subtotal, the HT and suppression patterns were similar, reaching suppression at 4 months, which was maintained during the study. The authors reported bone loss at the spine level of 2.6±1.9% per year, vs the 0.2±1% found in the control group of 15 normal. Paradoxically, if this loss were continued for ten years, it would be 26% in excess of the controls, a fact that has not been repeated in any other study. The radial bone density was normal. It is possible that in these results the large inter-individual variety of the bone parameters referred to is affected, as well as the use of two different techniques, such as SPA (single photon absorptiometry) and DXA.
The second longitudinal study is Mulller et al. [57]. They studied 15 premenopausal women and 10 postmenopausal women in T4 suppressive treatment for a variable period of 1.5 years. Of this group, 24 patients with CDT were re-evaluated with DXA with a follow-up interval of 1.5±0.5 years. They selected 15 matched controls in sex, menopausal status, age and BMI. They concluded that suppression of TSH was accompanied by non-significant reductions (2-5%) of lumbar BMD and femoral neck BMD, without any incident fractures. The decrease in BMD found is lower than the classic one described by Mazess, in which the increased risk of vertebral fracture increases 1.5-2 times for each standard deviation (DS) that decreases BMD58, which senses no effect to this level.
In the Fujiyama et al. series [59], 24 postmenopausal patients were described, divided into two groups, with and without TSH suppressor doses, with 12 patients with CDT each. Both groups had a similar bone loss rate: -0.849±0.605 in the suppressed ones, and -0.669±0.659 in the non-suppressed ones. On the other hand, Z-score values for lumbar and total body BMD were similar to those reported for healthy controls.
In 1996, Kung et al. [60] detailed a study in CDT-operated postmenopausal women who distributed in three subgroups with 15 patients in each: the first, in treatment with calcitonin; The second, with calcium alone; And the third group, with placebo without any treatment, which is the group we included in this review. Patients in this third group were followed for two years after the administration of levothyroxine and effective suppression of TSH (<0.03 mIU/L) postoperatively for approximately 9 years. They found a significantly superior bone loss at the lumbar, total hip, trochanter and Ward triangles (5.0%, 6%, 4.7%, 8.8%, respectively, p<0.05). However, when no fractures were found, they thought that the clinical importance of this bone decrease should be questioned.
Guo et al. [61] performed a prospective study in 23 postmenopausal women with intervened CDT and subsequent TSH suppression, followed by 2 years with bone densitometry and bone markers. Serum TSH levels were measured every 6-12 months to control TSH suppression. TSH levels were correlated with bone markers (osteocalcin, bone alkaline phosphatase and NTX). This group of postmenopausal women was compared with two other control groups (with and without suppressed TSH levels) who had primary hypothyroidism or Hashimoto's thyroiditis (n=41). They found that control patients had an increase in lumbar and femoral neck BMD and a decrease in bone markers, whereas patients with CDT had decreased bone markers without modifying BMD. Their results suggest that in postmenopausal women in T4 treatment, bone remodeling is related to the degree of TSH suppression, and that the decrease in T4 dose in those with suppressed TSH may induce a decrease in bone remodeling.
In our experience [55], we studied 14 premenopausal and 13 postmenopausal women with CDT and TSH suppression followed in our service since their total thyroidectomy with dual photon densitometry repeated for two years. Fifty percent of our patients had TSH below 0.1 mIU/L. The dose of LT4 showed a positive predictive value in each studied bone site which had been scarcely described. None of the bone and mineral parameters studied were correlated with bone mass, except for alkaline phosphatase at Ward's triangle level and ultradistal radius. This is consistent with normal BMD and bone remodeling values found in these patients with prolonged suppressive treatments. The suppressed patients showed a small reduction in BMD in 1/3 distal radius (Z-score -=0.77±0.98, CI 95: -1.11, -0.44), without differences between the pre and postmenopausal.
The study with longer duration of follow-up is that of Sijanovic et al. [62] These authors studied 19 premenopausal women with intervened CSD (mean age 39±8 years) who underwent T4 suppressive treatment for an average of 9.4 years. The prospective study with bone densitometry was performed in 4 years. They noted that after one year there was no significant bone loss in any region of the skeleton, and yet, after performing 3 measurements, at 4 years they recorded significant loss of BMD in the distal radius and not in other areas. They commented, surprisingly, that in their analysis there is a decrease although not significant of bone mass in other areas (data not given), so suppressive TSH therapy with thyroxine in a period of approximately 10 years may induce a risk of osteopenia In premenopausal women who reach menopause.
In a selective, rather large group of postmenopausal patients with papillary CDT, Sugitani et al. [63] analyzed the effect of post-operative suppressive TSH therapy on disease-free survival and its effects on BMD. Two groups were analyzed: 140 patients with suppression (mean TSH: 0.07±0.10 mIU/L, and 127 without suppression (mean TSH: 3.14±1.60 mIU/L) .In the non-suppressed group, 120 patients Postmenopausal women were followed for 5 years, showing a decrease in lumbar BMD subgroup of postmenopausal women over 50 years of age. TSH suppression had no significant effects on the prevention of relapses in papillary CDT, although most of its In the end, it is recommended that suppression of TSH, especially in patients with low risk and in elderly patients, is not indicated, taking into account that it has not been shown to decrease recurrences even in patients with high risk.
The longitudinal study by Karner et al. [64] was carried out in premenopausal women with CDT for one year. The duration of TSH suppression at the start of the study was 9.4±6.4 years, and therefore broad. BMD measurements were performed twice over a period of one year. Using single photon absorptiometry (SPA) for extremities and DXA, they found no decrease in BMD at the distal radius, or in lumbar and/or hip BMD. It is a longitudinal study of short duration, small number of subjects (19 premenopausal). Its main recommendation is to practice the bone densitometry study before initiating the suppressor therapy of TSH to identify the patients with high risk of osteoporosis.
More recently a study was published by Schneider R et al. [65] to evaluate the potential effects of LT4 suppressive treatment in 46 premenopausal women undergoing CDT on BMD and bone and muscle strength. It is a prospective, cohort-controlled, 1-year follow-up, in which bone mass is measured by dual lumbar and hip photometry, and bone and muscle strength using the polar stress index with dynamometry. They are simultaneously studying 23 premenopausal women undergoing LT4 replacement therapy. In both premenopausal populations, with suppressive treatment or with substitutive treatment, they do not find a decrease in axial BMD; The annual loss (g/cm2) in patients with CDT was not significantly different from those receiving LT4 replacement therapy (BMD -0.005 vs +0.004; BMD femoral neck: -0.005 vs +0.00; total hip BMD: +0.001 vs +0.003, respectively). The authors concluded that there is little evidence of adverse effects of levothyroxine on bone, and that premenopausal women with CDT may be at risk for lower BMD at the ultradistal radius. In spite of their null data in this sense, they attribute loss of unbalanced cortical BMD by trabecular augmentation, probably indicating a high endocortical trabecularization.
Kim et al. [66], in a one-year prospective study, found a decrease in bone mass that predominantly affects postmenopausal women compared to premenopausal women in their study. The annual postmenopausal loss was -2.1% in the lumbar spine, -2.2% in the femoral neck, -2.1% in the total hip, significantly higher than the premenopausal women (p<0.05 for all). Although the authors report that bone loss was primarily during the early post-thyroidectomy period, a longer study might confirm this.
Finally, Kim et al. [67] conducted a prospective 12-18 month study in 24 premenopausal women with CDT (6 hypoparathyroid glands) and 100 postmenopausal women (50 hypoparathyroid glands), concluding that they found no deleterious effect of suppressive therapy with T4, Even a protective effect in patients with post-operative hypoparathyroidism.
Risk factors in patients with CDT and TSH suppressor therapy
López Alvarez et al. [68] studied the risk factors involved in possible bone loss in 43 premenopausal and 53 postmenopausal women with TDC treated with suppressive thyroid hormones and followed up for an average of 75 months. Age, as a risk factor, and weight as a protective factor were the variables that most influenced BMD. No significant differences were found when comparing patients with normal concentrations of free thyroxine versus those who had them slightly elevated. In postmenopausal women, there was greater lumbar BMD in the group with adequate calcium intake (957 mg/day) compared to those who did not (855 mg/day) (p<0.05). At the level of the femoral neck and lumbar region, TSH, along with age and weight, were the variables that influenced the most. Gómez de Melo et al. [69] carried out another similar study in 109 postmenopausal women with CDT and suppressive treatment, in which they identified that, in the multivariate logistic regression analysis, the factors significantly related to lower BMD values were: low BMI and TSH; Do not find relation between the BMD and the average values of free T4. They suggest that TSH can have negative effects on BMD only when levels are suppressed.
In summary, most of what has been reported in relation to premenopausal women with CDT and suppressive therapy of TSH shows no deleterious effects on BMD in any anatomical site. In postmenopausal women with CDT and TSH suppression the studies are more heterogeneous, but, nevertheless, it must be pointed out that there are three studies, commented, with important population that refer to bone loss
Conclusions
In recent years, there have been important contributions to the better understanding of the regulation of the skeleton by HT and the hypothalamic-pituitary axis. The deiodination of the HT during its metabolism is considered an important determinant of the thyroid state at the circulating level and of the peripheral tissues. In bone, the activity of deiodinase D2 is involved in osteoblasts and in maintaining adequate mineralization and bone strength. Deiodinase D3 would intervene very early, at cartilage level favoring skeletal growth and development.
In subjects with subclinical hyperthyroidism other than CDT, controlled trials with a significant number of patients are considered necessary to evaluate the efficacy of normalizing TSH levels associated with fracture risk. The accumulated experience with the suppressive treatment of TSH in the CDT is configuring a therapeutic strategy with greater evidence for the treatment of patients with low risk and intermediate, in whom this approach would not be necessary. In contrast, patients at high risk could benefit; However, it is the elderly patients with high risk who usually have greater comorbidities and, in whom the indication will often have to be evaluated.
Referencias
1 Gogakos AI, Duncan Basset JH, Williams GR. Thyroid and bone. Arch Biochem Biophys. 2010;503:129-36. [ Links ]
2 Duncan Basset, Williams GR. Role of thyroid hormones in skeletal development and bone maintenance. Endocr Rev. 2016;37(2):135-87. [ Links ]
3 Waung JA, Duncan Basset, Williams GR. Thyroid hormone metabolism in skeletal development and adult bone maintenance. Trends Endocrinol Metab. 2012;23:155-62. [ Links ]
4 Wojcicka A, Duncan Basset, Williams GR. Mechanisms of action of thyroid hormones in the skeleton. Biochim Biophys Acta. 2013;1830:3979-86. [ Links ]
5 Schwartz CE, Stevenson RE. The MCT8 thyroid hormone transporter and Allan-Herndon-Dudley syndrome. Best Prac Test Clin Endocrinol Metab. 2007;21:307-21, [ Links ]
6 Bookout AL, Jeong Y, Downes M, Yu RT, Evans RM, Mangelsdorf DJ. Anatomical profiling of nuclear receptor expression reveals a hierarchical transcriptional network.Cell. 2006 Aug 25;126(4):789-99. [ Links ]
7 Williams GR, Bland R, Sheppard MC. Characterization of thyroid hormone (T3) receptors in three osteosarcoma cell lines of distint osteoblast phenotype:interaction among T3, vitamin D3 and retinoid signalling. Endocrinology. 1994;134:2375-85. [ Links ]
8 Saraiva PP, Teixeira SS, Padovani CR, Nogueira CR. Triiodothyronine (T3) does not induce Rankl expresión in rat Ros 17/2.8 cells. Arq Bras Endocrinol Metab. 2008;52:109-13. [ Links ]
9 Varga F, Spitzer S, Klaushofer K. Triiodothyronine (T3) and 1,25 dihydroxyvitamin D3 (1.25D3) inversely regulate OPG gene expression in dependence of the osteoblastic phenotype. Calcif Tissue Int. 2004;74:382-7. [ Links ]
10 Bochukova E, Schoenmaker N, Agostini M, Schoenmakers E, Rajanayagan O, Keogh JM, et al. Mutation in the thyroid hormone receptor alpha gene. N Engl J Med. 2012;366:243-9. [ Links ]
11 Kim TH, Joung JY, Kang M, Choi SK, Kim K, Jang JY, et al. A modest protective effect of thyrotropin against bone loss is associated with plasma triiodothyronine levels. PLoS One. 2015;10(12):e0145292. [ Links ]
12 Leader A, Ayzenfeld RH, Lishner M, Cohen E, Segev D, Hermoni D. Thyrotropin levels within the lower normal range are associated with an increased risk of hip fractures in euthyroid women, but no men, over the age of 65 years. J Clin Endocrinol Metab. 2014;99:2665-73. [ Links ]
13 Murphy E, Gluer CC, Reid DM. Felsenberg D, Roux C, Eastell R, et al. Thyroid function within the upper normal range is associated with reduced bone mineraldensity and an increased risk of non vertebral fractures in healthy euthryoid postmenopausal women. J Clin Endocrinol Metabol. 2010;95:3173-81. [ Links ]
14 Blum MR, Bauer DC, Collet TH, Fink Ha, Cappola AR, da Costa BR, et al. Subclinical thyroid diysfuncition and fracture risk: a meta-analsis. JAMA. 2015;313:2055-65. [ Links ]
15 Cummings S, Nevitt MC, Browner WS, Stone K, Fox KM, Kristine E, et al. Risk factors for hip fracture in white women. N Engl J Med. 1995;332:767-74. [ Links ]
16 Bauer DC, Ettinger B, Nevitt MC, Stone KL. Study of osteoprotic fractures research group. Risk for fractures in women with low serum levels of TSH. Ann Intern Med. 2001;134:561-8. [ Links ]
17 Baqi L, Payer J, Killinger Z, Hruzikova P, Cierny D, Susienkova K, et al. Thyrotropin versus thyroid hormone in regulating bone density and turnover in premenopausal women. Endocr Regul. 2010;44(2):57-63. [ Links ]
18 Valdiveloo T, Donnan PT, Cochrane L, Leese Gp. The thyroid epidemiology, audit and research study (TEARS): morbidity in patients with endogenous subclinical hyperthyroidism. J Clin Endocrinol Metab. 2011;96:1344-51. [ Links ]
19 Turner MR, Camacho X, Fischer HD, Turner MR, Camacho X, Fischer HD, et al. Levothyroxine dose and risk of fractures in older adults:nested case control study. BMJ. 2011:342:561-8. [ Links ]
20 Garin MC, Arnold AM, Lee JS, Robbins J, Cappola AR. Subclinical thyroid dysfunction and hip fracture and bone mineral density in older subjects: the cardiovacscular health study. J Clin Endocrinol Metab. 2014;99:2657-64. [ Links ]
21 Faber J, Galloc AM. Changes in bone mass during prolonged subclinical hyperthyroidism due to L thyroxine treatment: a meta analysis. Eur J Endocrinol. 1994;130:350-6. [ Links ]
22 Uzzan B, Campos J, Cucherat M, Nony P, Boissel JP, Perret GY. Effect on bone mass of long term treatment with thyroid hormones: a meta analysis. J Clin Endocrinol Metab. 1996;81:4278-89. [ Links ]
23 Wirth CD, Blum MR, da Costa BR, Baumgartner C, Collet TH, Medici M, et al. Subclinical thyroid dysfunction and the risk for fractures: a systematic review and meta analysis. Ann Intern Med. 2014;161:189-99. [ Links ]
24 Kim DJ, Khang YH, Koh JM, Shong YK, Kim GS. Low normal TSH levels are associated with low bone mineral density in healthy postmenopausal women. Clin Endocrinol. 2006;64;86-90. [ Links ]
25 Morris M. The association between serum thyroid-stimulating hormone in its reference range and bone status in postmenopausal American women. Bone. 2000:40:1128-34. [ Links ]
26 Tuchendler D, Bolanowski M. Assessment of bone metabolism in premenopausal femles with hyperthyroidism and hypothyroidism. Endokrynol Pol. 2013;64:40-4. [ Links ]
27 Basset JH, O´Shea PJ, Sriskantharaj S, Rabier B, Boyde A, Howell PG, et al. Thyroid hormone excess rather than thyrotropin deficiency induces osteoporosis in hyperthyroidism. Mol Endocrinol, 2007;21:1095-107. [ Links ]
28 Hwangbo Y, Kim JH, Kim SW, Park YJ, Park DJ, Kim SY, et al. High normal free thyroxine levels are associated with low normal trabecular bone scores in euthryoid postmenopausal women. Osteoporos Int. 2016;27:457-62. [ Links ]
29 Morris LG, Sikora AG, Tosteson TD, Davies L. The increasing incidence of thyroid cancer: the influence of access to care.Thyroid. 2013;23(7):885-9. [ Links ]
30 Vassart G, Dumont JE. The thyrotropin receptor and the regulation of thyrocyte function and growth. Endocr Rev. 1992;13:596-611. [ Links ]
31 McGriff NJ, Csako G, Gourgiotis L, Lori CG, Pucino F, Sarlis NJ. Effects of thyroid hormone suppression therapy on adverse clinical outcomes in thyroid cancer. Ann Med. 2002;34(7-8):554-64. [ Links ]
32 Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1-133. [ Links ]
33 Biondi B, Cooper DS. Benefits of thyrotropin suppression versus the risks of advere effects in differentiated thyroid cancer. Thyroid. 2010;20:135-45. [ Links ]
34 Wang Y, Smith AW, Palmer FL, Tuttle RM, Mahros A, Nixon IJ, et al. Thyrotropin suppression increases the risk of osteoporosiswsithout decreasing recurrence in ATA low and intermediate risk patients with differentiated thyroid carcinoma. Thyroid. 2015;25:300-7. [ Links ]
35 Turner MR, Camacho X, Fischer HD, Austin PC, Anderson GM, Rochon P, et al. Levothyroxine dose and risk of fractures in older adults: nested case-control study. BMJ. 2011;342:d2238. [ Links ]
36 Do Cao C, Wemeau JL. Risk benefit ratio for TSH suppressive Levothyroxine therapy in differentiated thyroid cancer. Ann Endocrinol. 2015;76:1S47-52. [ Links ]
37 Aboowara A, Quraishi A, Sapp IL, Alqambar MH, Sarie A, O Connell CM, et al. Prevalence of atrial fibrillation in patients taking TSH suppression therapy for management of thyroid cancer. Clin Invest Med. 2012;35:E152-6. [ Links ]
38 Flyn RW, Bonellie SR, Jung RT, McDonald TM, Morris AD, Leese GP. Serum thyroid stimulating hormone concentration and morbidity from cardiovascular disease and fractures in patients on longterm thyroxine therapy. J Clin Endocrinol Metab. 2010;95:186-93. [ Links ]
39 Samuels MH.Thyroid disease and cognition. Endocrinol Metab Clin North Am. 2014;43(2):529-43. [ Links ]
40 Jaracz J, Kucharska A, Rajewska-Rager A, Lacka K. Cognitive functions and mood during chronic thyrotropin-suppressive therapy with L-thyroxine in patients with differentiated thyroid carcinoma. J Endocrinol Invest. 2012;35(8):760-5. [ Links ]
41 Moon JH, Ahn S, Seo J, Han JW, Kim KM, Choi SH, et al. The effect of long-term thyroid-stimulating hormone suppressive therapy on the cognitive function of elderly patients with differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2014;99(10):3782-9. [ Links ]
42 Papaleontiou M, Hawley ST, Haymart MR. Effect of thyrotropin suppression therapy on bone in thyroid cancer patients. Oncologist. 2016;21(2):165-71. [ Links ]
43 Baliram R, Sun L, Cao J, Li J, Latif R, Huber AK, et al. Hyperthyroid associated osteoporosis is exacerbated by the loss of TSH signaling. J Clin Invest. 2012;122:3737-41. [ Links ]
44 Ross DS, Neer RM, Ridgway EC, Daniels GH. Subclinical hyperthyroidism and reduced bone density as a possible result of prolonged suppression of the ituitary thyroid axis with L thyroxine. Am J Med. 1987;82:1167-70. [ Links ]
45 Paul Tl, Kerrigan J, Kelly AM, Braverman LE, Baran DT. Long term L thyroxine therapy is associated with decreased hip bone density in premenopausal women. JAMA. 1988;259:3137-41. [ Links ]
46 Diamond T, Nery L, Hales A. A therapeutic dilemma: suppressive doses of thyroxine significantly reuce bone mineral measurements in both premenoausal and postmenopausal women wih thyroid carcinoma. J Clin Endocrinol Metab. 1991;72:1184-8. [ Links ]
47 Reverte JL, Holgado S, Alonso N, Salinas I, Granada ML, Sanmarti A. Lack of deleterious effect on bone mineral density of long term thyroxine suppressive therapy for differentiated thyroid carcinoma. Endocr Relat Cancer. 2005;12:973-81. [ Links ]
48 Kung AWC, Lorentz T, Tam SCF. Thyroxine suppressive therapy decreases bone mineral density in postmenopausal women. Clin Endocrinol. 1993;39:535-40. [ Links ]
49 Stepan JJ, Limanova Z. Biochemical assessment of bone losss in patients on long term thyroid hormone treatment,. Bone Miner. 1992;17:377-88. [ Links ]
50 Hawkins F, Rigopoulou D, Papapietro K, Lopez Alvarez MB. Spinal bone mass alter long-term treatment with L-Thyroxine in postmenopausal women with thyroid cancer and chronic lymphocytic tiroiditis. Calcif Tissue Int. 1994;54:16-9. [ Links ]
51 Franklyn JA, Betteridge J, Daykin J, Holder R, Oates GD, Parle JV, et al. Long-term thyroxine treatment and bone mineral density. Lancet. 1992;340:9-13. [ Links ]
52 De Melo TG, da Assumpcao LV, Santos Ade O, Zantut-Wittmann DE. Low BMI and low TSH value as risk factors related to lower bone mineral density in pstmenoausal women under levothyroxine herapy for differeniated thyroid carcinoma. Thyroid Res. 2015;8:7. [ Links ]
53 Tournis S, Antoniou JD, Liakou CG, Christodoulou J, Papakitsou E, Galanos A, et al. Volumetric bone mineral density and bone geometry assessed by peripheral quantitative computed tomography in women with differentiated thyroid cancer under TSH suppression. Clin Endocrinol. 2015;82:197-204. [ Links ]
54 Newman T, Browner W, Cumming S, Hulley S. Designing a new study: cross-sectional and case control studies. In: Hulley S, Cumming S, (eds.) Designing Clinical Research. Baltimore: Williams and Wilkings; 1988. p. 77-78. [ Links ]
55 Jodar E, López B, García L, Rigopoulou D, Martinez G, Hawkins F. Bone Changes in pre and postmenopausal women with thyroid cancer and levothyroxine therapy: Evolution of axial and appendicular bone mass. Osteoporos Int. 1998:8;311-6. [ Links ]
56 Pioli G, Pedrazzoni M, Palummeri E, Stanesi M, Del Frate R, Vescovi PP, et al. Longitudinal study of bone loss after thyroidectomy and suppressive thyroxine therapy in premenopausal women. Acta Endocrinol. 1992;126:238-42. [ Links ]
57 Muller CG, Bayley TA, Harrison JE, Tsang R. Possible limited bone loss with suppressive thyroxine therapy is unlikely to have clinical relevance. Thyroid. 1995;5(2);817-21. [ Links ]
58 Mazes RB. Peak bone mass, reference values, and T-scores. J Clin Densitom. 2001;4(1):73-7. [ Links ]
59 Fujiyama K, Kiriyama T, Ito M, Kimura H, Ashizawa K, Tsuruta M, et al. Suppressive doses of thyroxine do not accelerate age-related bone loss in late postmenopausal women. Thyroid. 1995 5(1):13-7. [ Links ]
60 Kung AWC, Yeung SSC. Prevention of bone loss induced by thyroxine suppressive therapy in postmenopausal women: The effect of calcium and calcitonin. J Clin Endocrinol Metab. 1996;81:1232-6. [ Links ]
61 Guo CY, Weetman AP, Eastell R. Longitudinal changes of bone mineral density and bone turnover in postmenoausal women on thyroxine. Clin Endocrinol. (Oxf) 1997;46:301-7. [ Links ]
62 Sijanovic S, Karner I. Bone loss in premenopausal women on long-term suppressive therapy with thyroid hormone. Medscape Womens Health. 2001;6(5):3. [ Links ]
63 Sugitani I, Fujimoto Y. Effect of postoperative thyrotropin suppressive therapy on bone mineral density in patients with papillary thyroid carcinoma: A prospective controlled study. Surgery. 2011;150:1250-7. [ Links ]
64 Karner I, Hrgović Z, Sijanović S, Buković D, Klobucar A, Usadel KH, et al. Bone mineral density changes and bone turnover in thyroid carcinoma patients treated with supraphysiologic doses of thyroxine. Eur J Med Res. 2005;10:480-8. [ Links ]
65 Schneider R, Schneider M, Reiners C, Schneider P. Effects of levothyroxine on bone mineral density, muscle force, and bone turnover markers: a cohort study. J Clin Endocrinol Metab. 2012;97(11):3926-34. [ Links ]
66 Kim MK, Yun KJ, Kim MH, Lim DJ, Kwon HS, Song KH, et al. The effects of thyrotropin-suppressing therapy on bone metabolism in patients with well-differentiated thyroid carcinoma. Bone. 2015;71:101-5. [ Links ]
67 Kim CW, Hong S, Oh SH, Lee JJ, Han JY, Hong S, et al. Change of bone mineral density and biochemical markers of bone turnover in patients on suppressive levothyroxine therapy for differentiated thyroid carcinoma. J Bone Metab. 2015;22:135-41. [ Links ]
68 Lopez Alvarez MB, Hawkins F, Rigopoulou D, Martinez G, Estenoz J, Orutño B, et al. Factores de riesgo y densidad mineral osea en mujeres en tratamiento prolongado con levotiroxina. Med Clin. 1999;112:85-9. [ Links ]
69 Gomes de Melo T, Montalli da Assumpcao LV, Oliveria Santo A, Engelbrecht D. Low BMI and low TSH value as risk factors related to lower bone mineral density in postmenopausal women under levothyroxine therapy for differentiated thyroid carcinoma. Thyroid Res. 2015;8:1-7. [ Links ]











 text in
text in 


