Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista de Osteoporosis y Metabolismo Mineral
versión On-line ISSN 2173-2345versión impresa ISSN 1889-836X
Rev Osteoporos Metab Miner vol.10 no.4 Madrid nov./dic. 2018 Epub 03-Abr-2023
https://dx.doi.org/10.4321/s1889-836x2018000400005
Original Articles
Factors secreted by bone cells induce intracellular calcium accumulation and cyclic AMP and activation of ERK 1/2 in prostate cancer cells; evaluation by fluorescence techniques in living cells
1Laboratorio de Fisiopatología Ósea - Instituto de Medicina Molecular Aplicada (IMMA) - Universidad San Pablo-CEU - Madrid (España)
2Departamento de Ciencias Médicas Básicas - Facultad de Medicina - Universidad San Pablo-CEU - Madrid (España)
3Departamento de Farmacología y Biología Química - Universidad de Pittsburgh - Pensilvania (Estados Unidos)
Objective:
To analyze in prostate tumor cells the effects caused by the secretome of bone cells on proliferation and on intracellular signaling pathways related to the progression of prostate cancer.
Materials and methods:
The effects of secreted factors present in conditioned media of pre-osteoblasts MC3T3-E1 and osteocytes MLO-Y4 on the proliferation of metastatic prostate adenocarcinoma cells PC-3 were characterized using trypan blue staining. The effects of media conditioned by MC3T3-E1 and MLO-Y4 cells on intracellular signaling molecules involved in the tumor progression of prostate adenocarcinoma cells PC-3 were observed by fluorescence techniques in living cells. The accumulation of intracellular calcium was studied using the fluorescent calcium indicator Fluo-4AM and the generation of cyclic AMP, and ERK 1/2 activation by Fluorescent Resonance Energy Transfer (FRET) using the EPAC and ERK-NES biosensors, respectively.
Results:
The stimulation of PC-3 cells with conditioned media of pre-osteoblasts MC3T3-E1 and osteocytes MLO-Y4 induced an increase in PC-3 adenocarcinoma cell proliferation. Media conditioned by bone cells also caused a transient increase in intracellular calcium accumulation and generation of cyclic AMP and increased ERK 1/2 activation.
Conclusions:
Bone cells secrete proliferation-activating factors and signaling pathways that favor the tumor progression of prostate cancer cells, suggesting that cross-communication between these cell types may favor the development of metastatic niches of prostate cancer in the bone.
Key words prostate cancer; secreted bone factors; intracellular signaling; fluorescence in living cells; calcium; cyclic AMP; ERK 1/2
Introduction
Bone metastasis is a frequent complication in advanced stages of patients with prostate cancer, one of the cancers with greater mortality and morbidity in developed countries1. Avoiding the different stages necessary for the tumor cell to abandon the primary tumor, migrate and establish itself in the bone microenvironment is one of the main strategies to prevent bone metastases2. The invasion of primary tumor cells into skeletal niches is associated with the activation of bone cells that release growth factors and cytokines, which in turn promote tumor growth in metastases. As a result, the so-called "vicious cycle" of bone metastases is generated, which varies the physiology of bone and alters bone remodeling3,4. In the case of bone metastases caused by prostate cancer, osteolytic and osteoblastic lesions are produced as a result of the activation of osteoclasts and osteoblasts respectively5. In bone metastasis processes, it has been observed that tumor cells are able to secrete factors such as tumor necrosis factor alpha (TNF-α), interleukin 11 (IL-11), matrix metalloprotease 1 (MMP1), Jagged1 and protein related to parathormone (PTHrP), which directly or indirectly activate osteoclasts, giving rise to osteoclast metastases6. Matrix degradation by osteoclasts releases transforming growth factor β (TGF-β) and insulin-like growth factor (IGF-1) that promote the survival of tumor cells7. In contrast, the secretion by tumor cells of other factors such as fibroblast growth factor (FGF) and bone morphogenetic proteins (BMPs) can stimulate osteoblast differentiation resulting in osteoblastic lesions8.
On the other hand, some studies have described the importance of second messengers and intracellular signaling pathways in the modulation of proliferation, malignancy and metastatic capacity of tumor cells. In this way molecules such as calcium, cyclic adenosine monophosphate (cyclic AMP) or kinases regulated by extracellular signals 1/2 (ERK 1/2), have been proposed as mediators and possible therapeutic targets in tumor progression and bone metastasis9-11.
Despite the existence of various observations analyzing the factors secreted by tumor cells that affect bone cells, there is little information on the factors secreted by osteoblasts and osteocytes that act on tumorigenic prostate cells. In particular, the effect of factors secreted by bone cells on signaling pathways and second relevant messengers in the mediation of processes of tumor progression and metastasis to bone in tumor cells of prostate is little known.
In this study we have used fluorescence techniques in living cells to analyze whether factors secreted by bone cells can modify signaling pathways and second messengers in prostate adenocarcinoma cells. Our observations show that factors secreted by osteoblasts and osteocytes can induce proliferation of prostate tumor cells associated with accumulation of intracellular cyclic AMP and calcium and activation of the ERK kinase. These results suggest the key role of bone factors in intracellular mechanisms relevant to tumor progression and bone metastasis.
Material and methods
Cell cultures
Human prostatic carcinoma cells derived from bone metastases (PC-3, ATCC: CRL-1435) were cultured in RPMI 1640, supplemented with 10% fetal bovine serum (FBS). The murine pre-osteoblastic cell line MC3T3-E1 (ATCC: CRL-2593) and murine osteocytic MLO-Y4 (generously donated by Lynda Bonewald) were cultured in DMEM with 10% FBS or α-MEM with 2.5% fetal serum from Ram (SCF) and 2.5% SFB, respectively. All cells were cultured in media containing penicillin (100 units/mL) and streptomycin (100 µg/mL) in a humidified incubator at 37°C and 5% atmospheric CO2. Conditioned media were obtained from PC3, MLO-Y4 or MC3T3-E1 cells cultured in α-MEM in the absence of serum for 24 h.
Transfections
For transient transfections, PC-3 cells were cultured on glass coverslips of 25 mm diameter for 12 h prior to transfection with FuGENE 6 (Roche Applied Science), which was performed in complete culture medium. After 24 h the cover slips were transferred in an Attofluor chamber (Invitrogen, Carlsbad, CA) with HEPES/bovine serum albumin solution (BSA) (pH=7.4) (HEPES 0.1% (w/v) ASB solution) for real-time fluorescence experiments.
Cell proliferation assay
The number of viable PC-3 cells stimulated with conditioned media of cells MC3T3-E1, MLO-Y4 or of the PC-3 itself was evaluated by the trypan blue exclusion test as previously described12.
Measurement of intracellular calcium
The accumulation of intracellular calcium was quantified with the calcium sensitive sensor Fluo-4/AM (Invitrogen, Carlsbard, CA) following the manufacturer's protocol as previously described13. Briefly, PC-3 cells were cultured on MatTek culture plates with 2 µM Fluo-4/AM in Hanks' balanced salt solution (Invitrogen) at 22°C for 45 min. The cells were washed three times in the Hanks' solution and incubated at 22°C for 30 min. The intracellular calcium quantifications were performed with the inverted fluorescence microscope Nikon A1s. The fluorescence levels were measured at intervals of 1 s to 20 min. At least 30-40 cells were evaluated under each condition. The reagents ionomycin (increases the entrance of calcium ions in the cells) 10 µM and EGTA (calcium chelator) 10 mM were used to obtain the maximum and minimum stimulation in each cell analyzed.
Fluorescent Resonance Energy Transfer (FRET): assessment of intracellular
PC-3 cells were transiently transfected with EPAC cyclical AMP biosensor14 or with the ERK phosphorylation biosensor, ERK-NES15. The generation of cyclic AMP and the activation by phosphorylation of ERK were evaluated by Energy Transfer by Fluorescent Resonance (FRET) as previously described16. The cells were cultured in Ibidi culture plates of 35 mm diameter and kept in FRET buffer solution (137 mM NaCl, 5 mM KCl, 1 mM CaCl 2, 1 mM MgCl 2, 20 mM HEPES, 0.1% bovine serum albumin, pH 7.4) where they were transiently transfected with constructs consisting of the fusion proteins: fluorescent protein cyan (CFP)-EPACyellow fluorescent protein (YFP) or by CFP-ERK-NES-YFP and which is activated by direct binding of cyclic AMP or by phosphorylation, respectively, undergoing conformational changes that result in variations in FRET responses. Quantifications were carried out on a Leica microscope equipped with a 40x objective of immersion oil, sequential records of the CFP and YFP fluorescence channels being made. The intensities of the fluorescence emission were determined at 535/15 nm (YFP) and 480/20 nm (CFP) with a long dichroic passage (DCLP) of 505 nm. The FRET signal was monitored as the emission index of YFP (FYFP) and CFP (FCFP). The results are shown as the normalized mean (nFRET) ± standard error.
Results
Soluble factors of MC3T3-E1 and MLOY-4 induce increased proliferation in human prostate adenocarcinoma cells PC-3
Previous studies suggest that the bone environment favors the stimulation of prostate cancer cells promoting the establishment of skeletal metastases17. To evaluate the effects of factors secreted by bone cells on prostate carcinoma cells, we first analyzed the actions of conditioned media of osteoblasts MC3T3-E1 and osteocytes MLO-Y4 on the proliferation of PC-3 prostate cancer cells. Both the conditioned means of osteoblasts MC3T3-E1 and those of MLO-Y4 were found to induce an increase in the proliferation of PC-3 cells after 3 days of stimulation compared to control conditioned media (of the PC-3 cells themselves) (Figure 1).
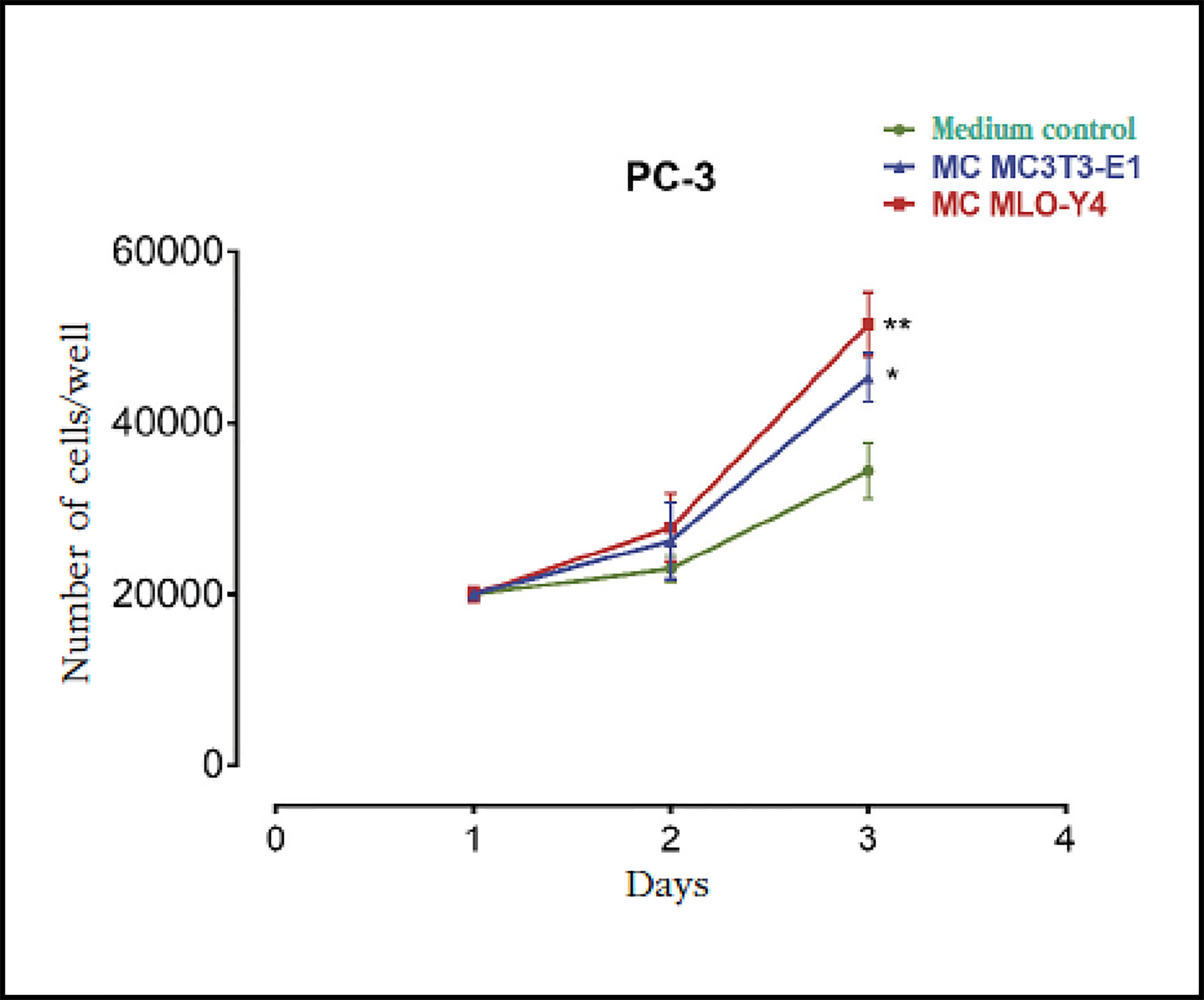
Figure 1 Factors secreted by osteoblasts MC3T3-E1 and osteocytes MLO-Y4 increase the proliferation of PC-3 prostate carcinoma cells. The PC-3 cells were incubated for 1-3 days with conditioned media (MC) obtained from MC3T3-E1 or MLO-Y4 and the number of cells was evaluated by trypan blue assay. The data shown are means ± standard error of 3 independent experiments *p<0.05; **p <0.01 vs. Conditionedmedium (MC) Control
Osteoblastic and osteoblastic bone soluble factors induce the formation of cyclic AMP and intracellular calcium release in human prostate adenocarcinoma cells PC-3
Next, the effects of conditioned media of bone cells in the activation of second messengers and signaling pathways related to tumor progression, metastasis and the activation of osteogenic responses9-11,18 were studied by means of fluorescence techniques in living cells. The conditioned media of osteoblasts MC3T3-E1 and osteocytes MLO-Y4 caused a rapid and transient increase in intracellular calcium concentration in prostate cancer cells PC-3 compared to stimulation with medium conditioned by the PC-3 cells themselves (Figure 2A-C).

Figure 2 Factors secreted by MC3T3-E1 and MLO-Y4 increase the intracellular calcium signaling of PC-3. We analyzed the effects of conditioned secreted factors obtained during 24 hours of MC3T3-E1 (A), MLO-Y4 (B) or PC-3 (C) in the intracellular calcium release of PC-3. The evaluation of intracellular calcium levels was performed by confocal fluorescence in living cells with the Fluo-4AM indicator as described in the text. The arrows indicate the moment of stimulation with conditioned means. The data shown are means ± standard error of 3 independent experiments
Similarly, the generation of cyclic AMP detected by FRET was stimulated by conditioned means of osteoblasts and osteocytes (Figure 3A-C). The levels of cyclic AMC did not vary when stimulating the PC-3 cells with conditioned media of PC-3 (data not shown).

Figure 3 Factors secreted by MC3T3-E1 and MLO-Y4 increase the cyclic AMP signaling of PC-3. (A) We analyzed the effects of conditioned secreted factors obtained during 24 hours of MC3T3-E1 and MLO-Y4 in the activation of PC-3 cyclic AMP. The evaluation of cyclic AMP was performed by confocal fluorescence in living cells with the CFPE-PACYFP sensor as described in the text. The arrows indicate the moment of stimulation with conditioned means. Forskolin was used to obtain maximum stimulation of cyclic AMP. The data shown are means ± standard error of 3 independent experiments. (B and C) Representative images of the fluorescence changes of the CFP and YFP fluorescent proteins of the EPAC cyclic AMP sensor in PC-3 cells after stimulation with conditioned medium of MC3T3-E1 or MLO-Y4 cells
Activation of the ERK 1/2 signaling pathway in human prostate adenocarcinoma cells PC-3 after stimulation of soluble bone factors
Phosphorylation of ERK 1/2 kinase, a protein directly involved in the proliferation of prostate tumor cells19, was also induced by conditioned media of osteoblasts MC3T3-E1 and osteocytes MLO-Y4 (Figure 4A and B). The conditioned medium of PC-3 cells, on the other hand, did not cause changes in the phosphorylation of ERK 1/2 of PC-3 cells (Figure 4B).

Figure 4 Factors secreted by MC3T3-E1 and MLO-Y4 increase the phosphorylation of the ERK 1/2 kinase of PC-3. We analyzed the effects of conditioned secreted factors obtained during 24 hours of MC3T3-E1 (A) or MLOY-4 (B) on phosphorylation of the ERK 1/2 kinase in PC-3. As a control, PC-3 cells were stimulated with conditioned medium of PC-3 cells. The evaluation of cyclic AMP was performed by confocal fluorescence in living cells with the CFPERK-NESYFP sensor as described in the text. The arrows indicate the moment of stimulation with conditioned means. The data shown are means ± standard error of 3 independent experiments
These results as a whole show that the factors secreted by bone cells modulate key signaling molecules in cellular processes such as the proliferation of prostate tumor cells.
Discussion
Our results show that metastatic prostatic adenocarcinoma cells increase their proliferation with factors secreted by both osteoblastic and osteocytic cells. In the case of bone metastases, it has been hypothesized that tumor cells are established in specific areas of bone such as the endosteal niche, the niche of hematopoietic stem cells and the vascular niche20. These niches are complex microenvironments in which factors that promote the physiological functions of the cells that compose them are secreted. It has been shown that increasing the number of these niches experimentally also increases the number of disseminated tumor cells of primary tumors21. These observations suggest that the same factors that maintain the correct functioning of the cells of the bone niches are able in turn to promote the establishment and growth of tumor cells in bone metastases. From this point of view, osteoblasts and osteocytes located near the surface would form part of the endosteal niche and may generate factors that promote the growth of prostate tumor cells in this niche.
There are several mechanisms that regulate the mitotic cycle of metastatic cells in bone, including regulatory processes of the immune system, angiogenesis, extracellular matrix, various factors and hormones, and intracellular processes22. Among these mechanisms, it was observed that the balance in the activation between 2 protein kinases activated by mitogens (MAP kinases), p38 and ERK 1/2 affects in a key way the mitosis of metastatic tumor cells23. When ERK 1/2 is activated in comparison with p38, cell proliferation is favored, and on the contrary the activation of p38 against ERK 1/2 induces a cellular quiescent state23. We have observed that pre-osteoblasts and osteocytes can send soluble factors that activate the ERK 1/2 kinase in PC-3 cells thus promoting the proliferation of tumor cells.
In addition, we have observed that factors secreted into the environment conditioned by pre-osteoblasts and osteocytes also caused a transient increase in intracellular calcium concentration and in the generation of cyclic AMP. Both second messengers can regulate processes of proliferation and tumor metastasis and have been proposed as possible therapeutic targets in several cancers9,10,24. Cyclic AMP can have positive or negative effects on the growth and survival of tumor cells depending on the cell type10. In tumors of epithelial origin such as prostate cancer, cyclic AMP seems to play a role in promoting oncogenesis by activating protein kinase A and other proteins activated below (for example, EPAC and CREB)25,26.
On the other hand, it has been shown that the increase in intracellular calcium concentration of extracellular origin is a factor that induces the proliferation of prostate cell lines of bone metastases (PC3 and C4-2B), but does not affect the proliferation of non-metastatic prostatic cell lines such as LNCaP9 cells. The increase in the concentration of calcium of extracellular origin causes PC-3 an increase in the expression of cyclin D1 (a regulatory protein of the cell cycle necessary in the proliferation), in the activation of Akt (protein required for the proliferation and tumor progression)27,28, and increases the binding capacity of tumor cells to substrate9. In addition, alterations in the gene expression of various calcium ion channels, such as TRP and Orai, have been associated with increases in calcium entry in prostate tumor cells that facilitate proliferation and resistance to apoptosis of those cells29,30.
Overall, these studies show the relevant function of the activation of the kinase ERK 1/2, calcium and cyclic AMP in the progression of prostate cancer. Although themodulation of these signaling pathways by factors secreted by bone cells has not been previously described, some studies have demonstrated the ability of resident bone cells to modulate the activity of tumor cells in metastatic niches. It has been observed that osteocytes mechanically stimulated by increased pressure caused by metastatic tumors induce growth and invasiveness of prostate tumors through the secretion of chemokine (C-C) ligand 5 (CCL5)31. Interestingly, the stimulation of cells of different types of cancer by CCL5 is able to increase the invasive and migratory capacity of tumor cells through mechanisms dependent on the intracellular mobilization of calcium32 or activation of the ERK kinase33,34. These observations suggest that CCL5 or other similar factors of the secret of bone cells could be responsible for the changes in signaling pathways of tumor cells that we have observed in the present study. On the other hand, previous publications have also demonstrated the key role of bone cells in promoting the activation of tumor cells and favoring metastatic processes based on direct bone cell-tumor cell contact through the activation of the Notch-Jagged signaling pathway35. Factors secreted by bone cells may mediate initial metastatic tumor recruitment and growth processes, where there is no direct contact between the tumor and the bone cells, while signaling pathways such as Notch-Jagged may regulate the interactions of the tumor. tumor in more advanced metastatic phases (in which the tumor does come into direct contact with bone cells).
Based on these investigations and our results, we propose that osteoblastic and osteocytic cells regulate the proliferation and activation of molecular mediators of tumor progression in metastatic prostate cancer cells by the secretion of soluble factors. We also suggest that the modulation of calcium intracellular mediators, cyclic AMP and ERK 1/2 by factors secreted by bone cells could be key in the establishment of bone metastases by prostate tumor cells.
Bibliografia
1 Wong MC, Goggins WB, Wang HH, Fung FD, Leung C, Wong SY, et al. Global incidence and mortality for prostate cancer: analysis of temporal patterns and trends in 36 countries. Eur Urol. 2016;70(5):862-74. [ Links ]
2 Kan C, Vargas G, Pape FL, Clézardin P. Cancer cell colonisation in the bone microenvironment. Int J Mol Sci. 2016;17(10):1-16. [ Links ]
3 Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004; 350(16):1655-64. [ Links ]
4 Guise TA, Mohammad KS, Clines G, Stebbins EG, Wong DH, Higgins LS, et al. Basic mechanisms responsible for osteolytic and osteoblastic bone metastases. Clin Cancer Res. 2006;12(20 Pt 2):6213-7. [ Links ]
5 Keller ET, Brown J. Prostate cancer bone metastases promote both osteolytic and osteoblastic activity. J Cell Biochem. 2004;91(4):718-29. [ Links ]
6 Ell B, Kang Y. SnapShot: bone metastasis. Cell. 2012;151(3):690-690.e1. [ Links ]
7 Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Rev Cancer. 2013;19(11):1423-37. [ Links ]
8 Suva LJ, Washam C, Nicholas RW, Griffin RJ. Bone metastasis: mechanisms and therapeutic opportunities. Nat Rev Endocrinol. 2011; 7(4):208-18. [ Links ]
9 Liao J, Schneider A, Datta NS, McCauley LK. Extracellular calcium as a candidate mediator of prostate cancer skeletal metastasis. Cancer Res. 2006;66(18):9065-73. [ Links ]
10 Fajardo AM, Piazza GA, Tinsley HN. The role of cyclic nucleotide signaling pathways in cancer: targets for prevention and treatment. Cancers (Basel). 2014;6(1):436-58. [ Links ]
11 McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F, et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta. 2007; 1773(8):1263-84. [ Links ]
12 Strober W. Trypan blue exclusion test of cell viability. Curr Protoc Immunol. 2001; Appendix 3: Appendix 3B. [ Links ]
13 Wang B, Ardura JA, Romero G, Yang Y, Hall RA, Friedman PA. Na/H exchanger regulatory factors control parathyroid hormone receptor signaling by facilitating differential activation of G(alpha) protein subunits. J Biol Chem. 2010;285(35):26976-86. [ Links ]
14 Nikolaev VO, Bunemann M, Hein L, Hannawacker A, Lohse MJ. Novel single chain cAMP sensors for receptor-induced signal propagation. J Biol Chem. 2004;279(36):37215-8. [ Links ]
15 Komatsu N, Aoki K, Yamada M, Yukinaga H, Fujita Y, Kamioka Y, et al. Development of an optimized back bone of FRET biosensors for kinases and GTPases, Mol Biol Cell. 2011;22(23):4647-56. [ Links ]
16 Alonso V, Ardura JA, Wang B, Sneddon WB, Friedman PA. A naturally occurring isoform inhibits parathyroid hormone receptor trafficking and signaling. J Bone Miner Res. 2011;26(1):143-55. [ Links ]
17 Karlsson T, Sundar R, Widmark A, Landstrom M, Persson E. Osteoblast-derived factors promote metastatic potential in human prostate cancer cells, in part via non-canonical transforming growth factor ? (TGF?) signaling. Prostate. 2018;78(6):446-56. [ Links ]
18 Thompson WR, Rubin CT, Rubin J. Mechanical regulation of signaling pathways in bone. Gene. 2012;503(2): 179-93. [ Links ]
19 Rodríguez-Berriguete G, Fraile B, Martínez-Onsurbe P, Olmedilla G, Paniagua R, Royuela M. MAP kinases and prostate cancer. J Signal Transduct. 2012;2012:169-170. [ Links ]
20 Ottewell PD. The role of osteoblasts in bone metastasis. J Bone Oncol. 2016;5(3):124-7. [ Links ]
21 Shiozawa Y, Pedersen EA, Havens AM, Jung Y, Mishra A, Joseph J, et al. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J Clin Invest. 2011; 121(4):1298-312. [ Links ]
22 Shaked Y, McAllister S, Fainaru O, Almog N. Tumor dormancy and the angiogenic switch: possible implications of bone marrow- derived cells. Curr Pharm Des. 2014;20(30):4920-33. [ Links ]
23 Aguirre-Ghiso JA, Ossowski L, Rosenbaum SK. Green fluorescent protein tagging of extracellular signal-regulated kinase and p38 pathways reveals novel dynamics of pathway activation during primary and metastatic growth. Cancer Res. 2004;64(20):7336-45. [ Links ]
24 Cui C, Merritt R, Fu L, Pan Z. Targeting calcium signaling in cancer therapy. Acta Pharm Sin B. 2017;7(1):3-17. [ Links ]
25 Caretta A., Mucignat-Caretta C. Protein kinase A in cancer. Cancers. 2011;3(1):913-26. [ Links ]
26 Borland G, Smith BO, Yarwood SJ. EPAC proteins transduce diverse cellular actions of cAMP. Br J Pharmacol. 2009;158(1):70-86. [ Links ]
27 Graff JR, Konicek BW, McNulty AM, Wang Z, Houck K, Allen S, et al. Increased AKT activity contributes to prostate cancer progression by dramatically accelerating prostate tumor growth and diminishing p27Kip1 expression. J Biol Chem. 2000;275(32):24500-5. [ Links ]
28 Chen H, Zhou L, Wu X, Li R, Wen J, Sha J, et al. The PI3K/AKT pathway in the pathogenesis of prostate cancer. Front Biosci. 2016;21:1084-91. [ Links ]
29 Monet M, Lehen'kyi V, Gackiere F, Firlej V, Vandenberghe M, Roudbaraki M, et al. Role of cationic channel TRPV2 in promoting prostate cancer migration and progression to androgen resistance. Cancer Res. 2010;700):1225-35. [ Links ]
30 Dubois C, Vanden Abeele F, Lehen'kyi V, Gkika D, Guarmit B, Lepage G, et al. Remodeling of channelforming ORAI proteins determines an oncogenic switch in prostate cancer. Cancer Cel. 2014;26(1):19-32. [ Links ]
31 Sottnik JL, Dai J, Zhang H, Campbell B, Keller ET, Tumor-induced pressure in the bone microenvironment causes osteocytes to promote the growth of prostate cancer bone metastases. Cancer Res. 2015;75(11):2151-8. [ Links ]
32 Singh SK, Mishra MK, Eltoum IA, Bae S, Lillard JW Jr, Singh R. CCR5/CCL5 axis interaction promotes migratory and invasiveness of pancreatic cancer cells. Sci Rep. 2018;8(1):1323. [ Links ]
33 Huang CY, Fong YC, Lee CY, Chen MY, Tsai HC, Hsu HC, et al. CCL5 increases lung cancer migration via PI3K, Akt and NF-kappaB pathways. Biochem Pharmacol. 2009;77(5):794-803. [ Links ]
34 Kato T, Fujita Y, Nakane K, Mizutani K, Terazawa R, Ehara H, et al. CCR1/CCL5 interaction promotes invasion of taxane-resistant PC3 prostate cancer cells by increasing secretion of MMPs 2/9 and by activating ERK and Rac signaling. Cytokine. 2013;64(1):251-7. [ Links ]
35 Sethi N, Dai X, Winter Cg, Kang Y. Tumor-derived JAGGED1 promotes osteolytic bone metastasis of breast cancer by engaging notch signaling in bone cells. Cancer Cell. 2011;19(2):192-205. [ Links ]
Received: July 05, 2018; Accepted: November 18, 2018











 texto en
texto en 


