Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista de Osteoporosis y Metabolismo Mineral
versão On-line ISSN 2173-2345versão impressa ISSN 1889-836X
Rev Osteoporos Metab Miner vol.11 no.1 Madrid Jan./Mar. 2019
https://dx.doi.org/10.4321/s1889-836x2019000100004
Originals
The determining role of a resorption marker, carboxyterminal telopeptide of collagen I, in assessing therapeutic compliance in patients treated with oral bisphosphonates
1Atención Primaria Barcelona Ciudad - Instituto Catalán de Salud - Barcelona (España)
2Grupo de Investigación en Enfermedades Prevalentes del Aparato Locomotor (GREMPAL) - Instituto de Investigación en Atención Primaria (IDIAP) Jordi Gol - Universidad Autónoma de Barcelona - Barcelona (España)
3Centro de Investigación Biomédica en Red de Fragilidad y Envejecimiento Saludable (CIBERFES) - Instituto de Salud Carlos III (ISCIII) - Barcelona (España)
4Departamento de Medicina Interna - Instituto Hospital del Mar de Investigaciones Médicas (IMIM) - Universidad Autónoma de Barcelona - Barcelona (España)
5Departamento de Ortopedia, Reumatología y Ciencias Músculoesqueléticas Nuffield - Instituto Nacional para la Investigación de la Salud (NIHR) - Unidad de Investigación Biomédica Musculoesquelética - Universidad de Oxford - Oxford (Reino Unido)
Objective:
It is estimated that in one year between 50-60% of patients treated with osteoporosis drugs are non-compliant. There are different indirect methods of assessing compliance. Our objective is to test a single determination of the carboxyterminal telopeptide of type I collagen (CTX) to assess compliance in patients treated with bisphosphonates, either on its own or together with the Morinsky-Green questionnaire.
Material and method:
A diagnostic assessment study was carried out in 10 centers in Catalonia. Through consecutive sampling, postmenopausal women with osteoporosis were selected and treated with the same antiresorptive drug in the last year. Those treated with a drug other than bisphosphonate, with cognitive impairment, terminal illness, advanced renal failure or fracture in the previous year, were excluded. Data were collected on the diagnosis of osteoporosis and type of treatment. Analysis was requested with CTX determination. As a gold standard, the medication possession rate (MPR) was used. Using the ROC curve methodology, the theoretical CTX cut-off point was established. Sensitivity, specificity and positive predictive values were calculated to estimate therapeutic compliance.
Results:
100 patients were included, of which more than half were being treated with alendronate. According to the MPR, 70% were compliant. The mean CTX value was 0.193±0.146 ng/ml. It was lower in the compliant patients. A value of 0.196 ng/ml was established as a cut-off point to assess compliance. The joint assessment of the CTX together with the Morinsky-Green questionnaire showed greater discriminatory capacity.
Conclusions:
Carrying out a single determination of CTX (<0.196 ng/ml) along with the Morinsky-Green questionnaire allows us to more accurately assess the therapeutic compliance in patients treated with bisphosphonates.
Key words: osteoporosis; bisphosphonates; therapeutic compliance; bone remodeling markers
INTRODUCTION
Osteoporosis is a metabolic disease characterized by low bone mass and microstructural deterioration of the bone tissue that leads to increased bone fragility. The main complication involves the appearance of fragility fractures1. Osteoporotic fractures are an important health problem2 associated with high healthcare costs3. To prevent the appearance of fractures, different drugs are available that act on bone metabolism and are associated with reduced fracture risk4. The most commonly used in Spain are bisphosphonates5. However, in order to observe this protective effect, adequate therapeutic compliance is required6. In osteoporosis, as in all chronic diseases, compliance is low. In a recent study conducted in Spain, the overall persistence per year after commencing osteoporosis drug is 47%, and at two years, close to 27%7.
Therefore, correctly assessing therapeutic compliance is necessary in our consultations to ensure an adequate effect in reducing the risk of fracture. Classically, self-administered surveys have been used to assess therapeutic compliance, such as Morisky-Green and Haynes-Sackett questionnaires, although the latter tends to overestimate compliance8. In recent years, thanks to health system computerization, it is possible in certain cases to have access to drug dispensing data, so that the medication possession rate (MPR) can be calculated. This is used in many pharmaco-epidemiological studies9-12, but not always available in day-to-day consultations.
Another possible way to assess compliance involves using bone remodeling markers, although there is little evidence in this regard and requires different determinations13. Determining carboxyterminal telopeptide of type I collagen (CTX) as a marker of resorption and of the amino terminal propeptide of type I procollagen (P1NP) as a formation marker is recommended14.
Our aim is to verify the usefulness of a single CTX determination to assess compliance in patients treated with bisphosphonates (the most prescribed drugs) for at least one year, in isolation or together with a classic therapeutic compliance questionnaire, such as Morisky- Green.
MATERIAL AND METHOD
Study design
Diagnostic validation study carried out in 9 urban primary care centers of the Catalan Health Institute in Barcelona and the Hospital del Mar, between January and December 2012. Accepting 95% confidence and assuming 55% of non-compliers a sample of 93 patients would detect a sensitivity of 80% with an accuracy of 10%.
Participants
Through consecutive sampling, all patients with postmenopausal osteoporosis and treatment with a drug for osteoporosis were selected at least during the last year to complete a total of 115 patients, to cover possible losses. Patients who were treated with an anti-resorptive drug different from an oral bisphosphonate, with cognitive impairment, terminal illness, or advanced chronic renal failure (glomerular filtration <35 ml/min), or who had presented a fracture in the year prior to inclusion were excluded.
Study variables
Information was collected on age, diagnosis of osteoporosis, study with bone densitometry and the presence of previous fractures. Regarding the osteoporosis treatment, the type of drug was collected, the dosage and the conditions of intake, as well as the use of calcium and/or vitamin D supplements. To assess the therapeutic compliance, the calculation of the MPR through pharmacy dispensing data in the year prior to inclusion. For its calculation, the following formula was used:
MPR = (number of presciptions collected in the last 12 months x days covered by each prescription)/365.
In accordance with available pharmaco-epidemiological studies, an MPR ≥0.8 is considered an indicator of therapeutic compliance15. The self-administered therapeutic compliance questionnaire of Morinsky-Green was also carried out.
CTX plasma determination was requested, measured by ELISA method, an electrochemiluminescence immunoassay (ECLIA) from Roche that uses two monoclonal antibodies, analyzed in the MODULAR ANALYTICS E170 autoanalyzer (Roche). The intraseries coefficient of variation value is 2.5% and the interseries value is 4.1%. The reference values of the test are: 0.01-1.008 ng/mL. Within a one-month period before the visit of the physician, the determination was carried out.
Statistic analysis
The characteristics of the studied population are described by univariate descriptive analysis, calculating mean and standard deviation for continuous variables and absolute frequency and percentage for categorical variables. The Chi-square test was used to compare proportions and the Student's T test was used to compare means.
The receiver operator characteristics (ROC) curve methodology was used to determine the area under the curve and the theoretical CTX cut-off point with the best sum of sensitivity and specificity. Sensitivity, specificity, and positive (VPP) and negative predictive values (NPV) were calculated to estimate therapeutic compliance by: 1) Morisky-Green questionnaire, 2) CTX cut-off point and 3) joint assessment of the Morisky-Green and the CTX value. To assess the concordance between the different systems to assess compliance, the Kappa coefficient was used.
All statistical tests were carried out with a confidence interval (CI) of 95%. The statistical package SPSS version 13.0 for Windows and EPIDAT (program for epidemiological analysis of data) Version 3.1 was used for all analyzes.
Ethical aspects
The study was carried out following Declaration of Helsinki principles, the standards of good clinical practice, and as proposed in the Guide of Good Practices in Health Science Research of the Catalan Institute of Health (Second edition)16. Informed consent was requested from patients. The contact and personal data of the participating patients were only accessible to the study investigators.
RESULTS
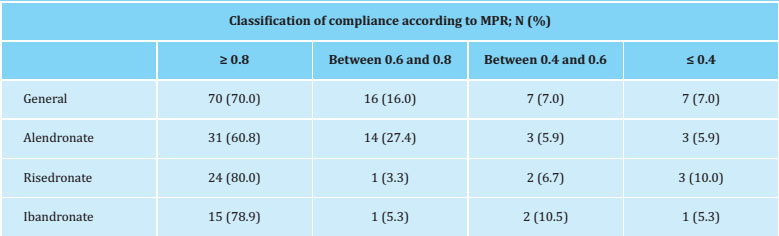
Of the 115 patients selected, 15 were excluded for taking a drug other than an oral bisphosphonate (9, strontium ranelate and 6, raloxifene). The baseline characteristics are shown in table 1, with a higher proportion of densitometries prior to treatment and a lower proportion of patients treated with alendronate in the group of compliant patients. In 11 women, different errors were identified in the taking of the medication (an error in the taking of the medication -not on an empty stomach- an error in the medication taking method -with milk- and 10 errors in the waiting time of fasting). As in these 11 cases, the MPR was <0.8 and, therefore, they were considered non-compliant, and thus not excluded from the analysis.
Table 1. Baseline characteristics in the total number of patients and according to treatment compliance

CaD: calcium and vitamin D supplements.
The therapeutic compliance valued by the MPR was 70% (Table 2), with no differences in the proportion of compliers, according to whether the treatment was weekly or monthly (68.2% vs. 73.5%, p=0.580). The compliance assessed by the self-administered Morinsky-Green questionnaire was 73%, with a moderate agreement compared to the MPR assessment (Kappa coefficient=0.436).
The mean value of the determination of CTX was 0.193±0.146 ng/ml (median=0.158 ng/ml), with lower patients compared to non-compliant patients (0.182±0.143 ng/ml vs. 0.2190±0.152 ng/ml; p=0.247). A cut-off point of CTX of 0.196 ng/ml was the one that presented a better sensitivity and specificity for the diagnosis of therapeutic compliance. Considering this CTX value, the therapeutic compliance was 64%, with a low concordance compared to the MPR assessment (Kappa coefficient=0.234). When considering the result of the Morinsky-Green questionnaire together with the value of the CTX, compliance was 51%, with a moderate agreement (Kappa coefficient=0.415).
Table 3 shows the values of sensitivity, specificity and predictive values of the different forms used to estimate therapeutic compliance. The area under the ROC curve (95% CI) for the Morisky-Green questionnaire was 0.7119 (0.6127-0.8111), and 0.6238 (0.5185-0.7291) for the evaluation by a CTX cut-off of 0.196 ng/ml (Figure 1). When considering the result of the Morisky-Green questionnaire together with the value of the CTX, the area under the ROC curve was 0.7452 (0.65730.8332), somewhat higher than if we only consider the Morisky- Green result (p=0.622) (Figure 2).
Table 3. Sensitivity values, specificity and predictive values for the different tools to estimate therapeutic compliance (95% CI)

VPP: positive predictive value; NPV: negative predictive value; MG+CTX: Morinsky-Green and CTX.
DISCUSSION
In our sample of patients treated with the same bisphosphonate for at least the last year, a CTX determination of less than 0.196 ng/ml is an indicator of therapeutic compliance in the last year, with a moderate discriminating capacity, lower than the discriminative capacity of the Morisky-Green survey. Their joint assessment (CTX <0.196 ng/ml and Morisky-Green) improves the discriminative capacity, being a good option to assess the therapeutic compliance in the consultations. In a recent consensus document13, the initial and three-month determination of bone remodeling markers (CTX and P1NP) was recommended to assess non-compliance based on the observed change (decrease of 56% of CTX and 38% of P1NP). But this requires two determinations of two markers, which are not always accessible for primary care laboratories. In addition, it does not allow for assessing compliance in those patients who have already started treatment and there is no baseline available, nor does it allow analyzing noncompliance over time.
Their specific determination, along with the administration of a classic therapeutic compliance questionnaire, that of Morisky-Green, present the best sensitivity and the best negative predictive value for therapeutic compliance.
In our sample, the observed therapeutic compliance (measured according to the MPR) was high, 70%, much higher than that observed in our environment by different observational studies7. One of the possible explanations is that our study was not designed to assess the proportion of therapeutic compliance in our population and, therefore, random sampling was not carried out. In addition, more than half of the patients included had a previous fracture, although there were no significant differences in the percentage of patients with previous fractures between compliant and noncompliant subjects. The presence of previous fractures is associated with higher rates of therapeutic compliance11.
One of every ten patients errors was observed in the correct way of taking the medication, a fact that in itself implies therapeutic noncompliance. These patients were not excluded ROC from the analysis since in all cases 1.0 the MPR was less than 0.8 and all 0.9 would be classified as non-compliant. As expected, the value of the CTX in these cases was not diminished since the absorption of the drug would be diminished. In the event that the MPR had been equal to greater than 0.8, the patient would have been considered as a non-compliant patient. As this situation has not occurred, 0.1 they have not been excluded from the study. Clear and concise information about the drug´s administration is required, as well as ensuring correct understanding of it, since an incorrect intake considerably decreases the absorption of the active principle and, therefore, the expected anti-fracture effect.
Unlike what was observed in previous studies carried out in the primary care field in Spain, where the diagnosis of osteoporosis was between 60-70%17,18 and the densitometry before diagnosis was approximately 65%17-19, in our study, both records were greater than 90%. This greater registry can be explained in part to a better registry of diseases and results over time, and to the fact that the patients included were assigned to doctors more aware of osteoporosis. This greater awareness of the professional with the condition could explain, in part, higher observed rates of compliance than that described in other population-based studies7,1.
One of this study´s limitations is the way in which therapeutic compliance is valued through pharmacy billing data since they are not a direct indicator that the patient actually takes the medication, but exclusively that it withdraws from the medication. pharmacy. In the absence of direct methods to assess compliance, this is the most approximate and recommended measure to assess compliance.
Any patient with an MPR of less than 80% has been considered non-compliant, but not whether the non-withdrawal of medication occurred in the first or last months of the period prior to the CTX determination. This fact could have an impact in the CTX value.
Another limitation is that, once a CTX cut-off point is available to assess compliance, another patient sample should be checked to confirm that similar results are observed.
As a strength, the fact that it is a single CTX determination and that can be carried out at any time, together with the completion of a compliance questionnaire, facilitates better compliance assessment of patients treated with bisphosphonates, although they have been taking it for a long time.
REFERENCES
1 Consensus NIH. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285:785-95. [ Links ]
2 Cauley JA, Wampler NS, Barnhart JM, Wu L, Allison M, Chen Z, et al. Incidenceof fractures compared to cardiovascular disease and breast cancer: the Women’sHealth Initiative Observational Study. Osteoporos Int. 2008;19:1717-23. [ Links ]
3 Caeiro JR, Bartra A, Mesa-Ramos M, Etxebarría Í, Montejo J, Carpintero P, et al. Burden of first osteoporotic hip fracture in Spain: a prospective, 12-month, observational study. Calcif Tissue Int. 2017;100:29-39. [ Links ]
4 Nogués X, Martinez-Laguna D. Update on osteoporosis treatment. Med Clin (Barc). 2018;150:479-86. [ Links ]
5 Martín-Merino E, Huerta-Álvarez C, Prieto-Alhambra D, Álvarez-Gutiérrez A, Montero-Corominas D. Secular trends of use of anti?osteoporotic treatmentsin Spain: A populationbased cohort study including over 1.5 million people and more than 12 years of follow-up. Bone. 2017;105:292-8. [ Links ]
6 Soong Y-K, Tsai K-S, Huang H-Y, Yang R-S, Chen J-F, Wu PC-H, et al. Risk of refracture associated with compliance and persistence with bisphosphonate therapy in Taiwan. Osteoporos Int. 2013;24:511-21. [ Links ]
7 Reyes C, Tebe C, Martinez-Laguna D, Ali MS, Soria-Castro A, Carbonell C, et al. One and two-year persistence with different anti-osteoporosis medications: a retrospective cohort study. Osteoporos Int. 2017;28:2997-3004. [ Links ]
8 Carbonell Abella C, Guañabens Gay N, Regadera Anechina L, Marín Rives JA, Taverna Llauradó E, Ayechu Redín MP. Analysis of therapeutic compliance in women with osteoporosis. Reumatol Clin. 2014;7:299-304. [ Links ]
9 Cheng L, Durden E, Limone B, Radbill L, Juneau PL, Spangler L, et al. Persistance and compliance with osteroporosis therapies among women in a commercially insured population in the United States. J Manag Care Spec Pharm. 2015;21:824-33. [ Links ]
10 Carbonell-Abella C, Pages-Castella A, Javaid MK, Nogues X, Farmer AJ, Cooper C, et al. Early (1-year) discontinuation of different anti?osteoporosis medicationscompared: a population-based cohort study. Calcif Tissue Int. 2015;97:535-41. [ Links ]
11 Klop C, Welsing PMJ, Elders PJM, Overbeek JA, Souverein PC, Burden AM, et al. Long-term persistence with anti-osteoporosis drugs after fracture. Osteoporos Int. 2015;26:1831-40. [ Links ]
12 Karlsson L, Lundkvist J, Psachoulia E, Intorcia M, Ström O. Persistence withdenosumab and persistence with oral bisphosphonates for the treatment ofpostmenopausal osteoporosis: a retrospective, observational study, and a meta-analysis. Osteoporos Int. 2015; 26:2401-11 [ Links ]
13 Diez-Perez A, Naylor KE, Abrahamsen B, Agnusdei D, Brandi ML, Cooper C, et al. International Osteoporosis Foundation and European Calcified Tissue Society Working Group. Recommendations for the screening of adherence to oral bisphosphonates. Osteoporos Int. 2017; 28:767-74. [ Links ]
14 Vasikaran S, Eastell R, Bruyère O, Foldes AJ, Garnero P, Griesmacher A, et al. Markers of bone turnover for the prediction of fracture risk and monitoringof osteoporosis treatment: a need for international reference standards. Osteoporos Int. 2011;22:391-420. [ Links ]
15 Caro JJ, Ishak KJ, Huybrechts KF, Raggio G, Naujoks C. The impact of compliance with osteoporosis therapy on fracture rates in actual practice. Osteoporos Int. 2004;15:1003-8. [ Links ]
16 Ariza A, Bosch X, Campos E, Vinyes J, Violan C, Visa J, et al. Guia de bona pràctica en la recerca en ciències de la Salut de l’ICS. 2015. [ Links ]
17 Martinez-Laguna D, Arias-Moliz I, Soria-Castro A, Estrada-Laza P, Coderch M, Nogués X, et al. Riesgo de fractura según FRAX®, hipovitaminosis D, y calidad devida en una población con fractura osteoporótica atendida en Atención Primaria: descriptiva basal de la cohorte VERFOECAP. Rev Osteoporos Metab Miner. 2011;3:157-64. [ Links ]
18 Martinez-Laguna D, Sancho-Almela F, Cano-Collado E, Gardeñes-Moron M, Morró J, Cos X. Uso adecuado en Atención Primaria de los fármacos antirresortivos frente a la osteoporosis. Rev Osteoporos Metab Miner. 2011;3:77-83. [ Links ]
19 Arana-Arri E, Gutiérrez-Ibarluzea I, Gutiérrez Ibarzabal ML, Ortueta Chamorro P, Giménez Robredo AI, Sánchez Mata AM, et al. Evidence based comparative analysis for managing osteoporosis in a primary health care setting. Aten Primaria. 2008;40:549-54. [ Links ]
Received: September 15, 2018; Accepted: October 28, 2018











 texto em
texto em