Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista de Osteoporosis y Metabolismo Mineral
versão On-line ISSN 2173-2345versão impressa ISSN 1889-836X
Rev Osteoporos Metab Miner vol.11 no.2 Madrid Abr./Jun. 2019 Epub 20-Jan-2020
https://dx.doi.org/10.4321/s1889-836x2019000200005
ORIGINALS
Osteogenic cells affected by soluble tumor factors contribute to bone pre-metastatic niche formation
1Laboratorio de Fisiopatología Ósea - Instituto de Medicina Molecular Aplicada (IMMA) - Universidad San Pablo-CEU - Alcorcón - Madrid (España)
2Departamento de Ciencias Médicas Básicas - Facultad de Medicina - Universidad San Pablo-CEU - Alcorcón - Madrid (España)
Objective:
To analyze the effect of the secrets of solid organotropic tumors towards bone in osteogenic, osteoblastic and osteocytic lineage cells, in the expression of genes related to bone metabolism.
Material and method:
We characterize the changes in gene expression by quantitative real-time PCR of the OPG/RANKL axis, as well as other genes related to osteoblastic differentiation such as Runx2 and osteocalcin, induced by the conditioned means of prostate tumor cells, breast and melanoma in pre MC3T3-E1 osteoblasts and murine MLO-Y4 osteocytes or in human osteoblasts, as appropriate by species.
Results:
Stimulation of osteocitic cells with conditioned means of melanoma or prostate adenocarcinoma cells induced an increase in OPG and RANKL gene expression, with the OPG/RANKL ratio being increased. Only the secretome of prostate adenocarcinoma cells altered the expression of Runx2 in osteocytes. Conditioned media of breast cancer cells only modified the expression of RANKL in osteoblast cells, with a decrease in OPG/RANKL ratio.
Conclusion:
Soluble tumor factors have osteocitic cells as their cellular target, favoring the induction of a pre-metastatic bone niche by modifying the OPG/RANKL ratio in the bone environment, and, thus, the progression of bone organotropic tumors such as melanoma and prostatic adenocarcinomas.
Key words bone organotropic tumors; soluble tumor factors; pre-metastatic bone niche; bone metastases; osteocytes and osteoblasts
INTRODUCTION
The appearance of metastatic disease seriously threatens the survival rate of patients who develop a tumor. Certain types of tumors have been found to present a high tendency to colonize specific organs. From the hypothesis formulated by Paget (“seed-and-soil”)1, few studies have deciphered the regulatory mechanisms of metastatic organotropism. Initial studies focused on the function of the intrinsic properties of the tumor cell, such as gene expression and colonization regulation pathways, in the direction of organotropism2-4.
Bone is an organ frequently infiltrated by the metastatic spread of solid tumors5,6. The appearance of metastatic disease is a serious threat in the survival rate of patients who develop a tumor. From 65-80% of subjects with prostate cancer or metastatic breast present skeletal complications5. The study of bone metastases has mainly focused on the interaction of the tumor cell with the bone, once the metastasis has been established, ignoring the subclinical stages of the process that occurs previously. The establishment of tumor cells in the bone microenvironment alters the balance of the bone remodeling process between bone formation, induced by osteoblasts, and osteoclast-mediated resorption. Consequently, the survival and proliferation pathways of tumor cells are favored, inducing the formation of "a vicious cycle of bone metastases"7.
Though not exclusive, tumors cause two different types of skeletal lesions. The most common form, represented by breast cancer is the osteolytic lesion associated with an alteration of bone remodeling with an increase in osteoclastic activity and subsequent osteolysis8-11.
A second type of so-called osteoblastic lesions is characterized by high bone remodeling with an increase in osteoblast activity with an increase in osteoid and mineralization rate. These areas of de novo-formed bone in areas of metastasis are called osteoesclerotic lesions, which are usually weak and unstable, with a tendency to break. This type of lesions is characteristic of prostate cancer. However, the existence of a osteoclast mediated component is currently recognized as a previous step for the establishment of osteoblastic lesions12,13.
Recent studies have described pro-metastatic changes in organs where metastases will later appear. Such changes induce the formation of pre-metastatic niches that favor the implantation of tumor cells in target or gans14,15
The cellular complexity of the bone (osteocytes, osteoblasts, osteoclasts, bone covering cells, endothelial cells and hematopoietic tissue), as well as the functions they carry out in regulating the metabolism and bone remodeling, raises the possibility that the formation of the pre niche-metastatic bone is the result of a complex network of combined and sequential modifications and alterations of all these cells16.
Despite the existence of some observations analyzing the effect of the factors secreted by tumor cells that affect the viability of bone cells17, the existence of common mechanisms or changes in bone cells induced by solid tumors with high organotropism is unknown. He made the bone as a metastatic target organ.
In this study, we have analyzed the changes in the transcriptional profile of cytokines related to bone metabolism in osteoblastic and osteocitic cells, induced by soluble tumor factors of breast, prostate and melanoma tumor cells. Our observations show that these factors significantly modify the transcriptional profile of osteocytes. These results suggest a relevant role of osteocytes as the initial inducing cell in the formation of the pre-metastatic bone niche.
MATERIAL AND METHOD
Cell cultures
The MC3T3-E1 murine pre-osteoblastic cell lines (ATCC: CRL-2593) and MLO-Y4 murine osteocitic cells (donated by Lynda Bonewald) were cultured in DMEM with 10% bovine fetal serum (SFB) or α-MEM with 2.5% fetal sheep serum (SCF) and 2.5% SFB, respectively. All cells were cultured in media containing penicillin (100 units/mL) and streptomycin (100 μg/mL) in the humidified incubator at 37°C and 5% atmospheric CO2.
The hFOB 1.19 human osteoblastic continuous line (ATCC® CRL-11372TM) was grown in a 1:1 mixture of Ham's F12 and DMEM with 2.5 mM L-glutamine, 0.3 mg/mL G418 and 10% SFB in the humidified incubator at 34°C and 5% atmospheric CO2.
We use the TRAMPC-1 mouse prostate adenocarcinoma (ATCC® CRL-2730TM), murine melanoma B16 (ATCC® CRL-6323) and human breast cancer (ATCC® MDA-MB-231 HTB-26) lines to obtain of tumor secretoma. These cells were cultured in DMEM culture medium supplemented with penicillin (100 U/ml), streptomycin (100 μg/ml), 1% glutamine and 10% SFB. When the culture reached confluence, they were washed with a phosphate solution (PBS). Next, tumor secrets and bone conditioned media used for stimulation of previously activated tumor cells with soluble bone factors were obtained..
To obtain the conditioned media of both tumor cells and bone cells, the cell lines were grown to confluence with α-MEM culture medium supplemented with penicillin and 0% streptomycin of SFB, after 24 hours of incubation, the media were collected and cell debris and dead cells were removed by centrifugation (5,000 rpm, 10 minutes).
To avoid any type of cross-biological reactivity of cytokines of one species in cells of another species18 and reproduce as faithfully as possible the communication of tumor cells with bone cells, our in vitro experimental models were designed according mainly to the criteria of species. In this way, the effects of the tumor secretoma of the human breast cancer line on the human osteoblastic line were evaluated. There is currently no well characterized osteocitic continuous line. The effects of the secrets of prostate and mouse melanoma tumor lines were studied on bone murine lines (osteoblastic, MC3T3, and osteocitic, MLOY4). Figure 1 shows the protocol used to obtain conditioned media and its stimulation in the different 25% cell lines.

Figure 1 Graphical representation of the work protocol used to obtain pre-treated or not conditioned means (MC). Untreated MLO-Y4, MC3T3-E1, human osteoblasts, B16, TRAMP-C1 or MDA-231 MCs were obtained after 24 h of culture in 0% SFB β-MEM and were used to stimulate the different cell lines The pre-treated MCs were obtained after 24 h cultivation of the B16 or MDA-231 with MC of MLO-Y4, MC3T3-E1 or human osteoblasts followed by a wash with PBS and 24 h of culture with 0% SFB β-MEM. Finally, a cell lysate was obtained which was analyzed by PCR
Cell viability assay
The number of viable bone cells MC3T3, MLOY4 and hFOBs stimulated or not with 25% tumor secretoma was evaluated by the trypan blue exclusion test as previously described19.
Gene expression studies by real-time PCR
Total RNA was extracted from cell cultures by the Trizol method. CDNA synthesis was performed using the reverse transcriptase of the avian myeloblastosis virus (Promega) and random hexamer primers. Real-time PCR was carried out in the ABI PRISM 7500 system (Applied Biosystems) using Sybr premix ex Taq (Takara, Otsu, Japan) and specific primers of each gene (Table 1). All results were expressed in number of mRNA copies calculated for each sample using the cycle threshold value (Ct). The relative gene expression is represented as: 2ΔΔCt, where ΔΔCt = ΔΔCtgen target - ΔΔCt18S/GAPDH. The change in the number of times with respect to treatment is defined as the expression compared to the control, calculated as 2-ΔΔCt, where ΔΔCt = ΔCreating - ΔCcontrol. The specificity of the amplicon was confirmed as the presence of a single amplification after melting curve analysis. The results shown correspond to the average of at least 3 independent experiments in triplicate.
RESULTS
Communication by soluble factors between tumorigenic melanocytes and bone cells regulates the expression of bone remodeling genes in osteocytes
In order to study the communication between tumor cells and bone cells in the formation of pre-metastatic bone niches, mouse or human bone cells were stimulated with the secrets of different tumor cells with organotropism to bone tissue, which had previously been stimulated or not with osteomas or osteoblast secrets (Figure 1).
First, we corroborate that the conditioned tumor media of the TRAMPC-1 or B16, that is to say tumor secretoma, does not affect the viability of the osteoblast cells, MC3T3, and osteocitic, MLOY4 after 24 h of stimulation (Figure 2). However, stimulation of MLO-Y4 osteocytes with secrets obtained from conditioned media of B16 melanoma cells induced overexpression of the OPG gene with a net increase in the OPG/RANK-L ratio, without significantly affecting the gene expression of RANK-L, nor to the transcription factor Runx2 (Figure 3A-D). In contrast, the secrets of B16 melanoma cells, which had previously been exposed to conditioned media of MLO-Y4 cells, caused an increase in the expression of RANK-L and a decrease in the OPG/RANK-L ratio. No changes were observed in the expression of OPG or Runx2 with respect to conditioned media without pre-stimulation (Figure 3A-D). These data suggest that melanoma tumor cells, which have been exposed to osteocyte secretoma, secrete soluble factors that cause a bone remodeling gene augmentation response, particularly increasing the expression of the osteoclastogenic factor RANK-L in osteocytes.
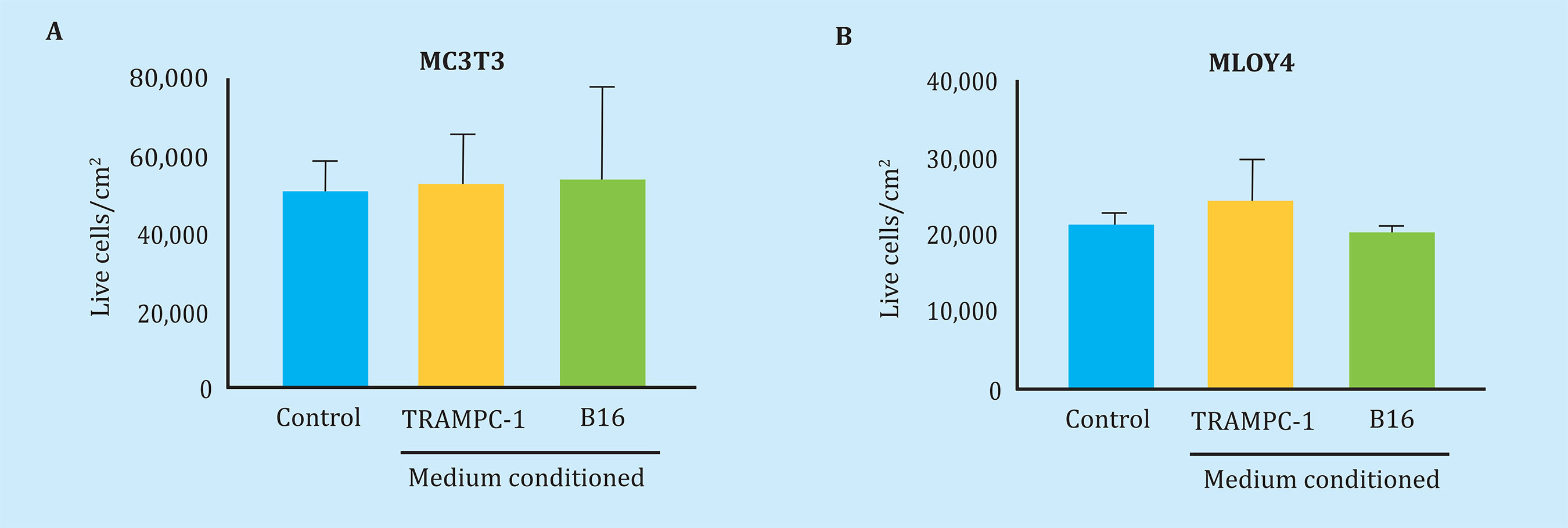
Figure 2 Soluble factors secreted by mouse tumorigenic melanocytes (B16) or mouse prostate tumor cells (TRAMPC-1) do not affect the viability of pre-osteoblastic murine bone cells (MC3T3-E1) or osteocitic (MLO-Y4) data. Represented as the mean ± SEM of 2 experiments in triplicate

Figure 3 Soluble factors secreted by tumorgenic melanocytes (B16) modify gene expression of bone remodeling in osteocytes (MLO-Y4) but not in pre-osteoblasts (MC3T3-E1). The expression of OPG (A, E), RANK-L (B, F), OPG/RANK-L ratio (C, G) and Runx2 (D, H) was evaluated by real-time PCR after stimulating 24 h MLO-Y4 and MC3T3-E1 with conditioned medium of B16 pre-treated or not. Data are represented as the mean ± SEM of three independent experiments in triplicate. *p<0.05 vs control, **p<0.01 vs control
Interestingly, these effects did not reproduce in MC3T3-E1 osteoblastic cells, in which the conditioned media of B16 cells did not cause gene overstimulations (Figure 3E-H).
Communication by soluble factors between tumor cells of the breast and bone cells regulates the expression of remodeling genes in osteblasts
We then analyze the effects of cells of another type of tumor that metastasize to bone, such as breast cancer5, on the gene expression of bone cells. In this case, we examine the effects of the secretome of the human tumor line of breast cancer MDA-MB-231 on human osteoblasts (Figure 1). The conditioned media of MDA-MB231 cells was found to cause an increase in the expression of RANK-L and a decrease in the OPG/RANK-L ratio, without affecting the expression of OPG or Runx2 in human osteoblasts (Figure 3A -D). The effects on gene expression of osteoblasts were similar using the secretomes of MDA-MB-231, both pre-treated and not pretreated with conditioned media of human osteoblasts (Figure 4A-C).

Figure 4 Soluble factors secreted by breast cancer tumor cells (MDA-MB-231) modify bone remodeling gene expression in human osteoblasts (hFOB 1.19). The expression of OPG (A), RANK-L (B), OPG/RANK-L (C) and Runx2 (D) ratio was evaluated by real-time PCR after 24 h stimulation of human osteoblasts with conditioned medium of pre-MDA treated or not. Data are represented as the mean ± SEM of three independent experiments in triplicate. *p<0.05 vs control
Communication through soluble factors between tumorous prostate cells and bone cells regulates the expression of remodeling and osteogenic genes in osteocytes
We then attempted to check if prostate tumor cells, potentially capable of metastasizing to bone20,21, also established communication with bone cells through soluble factors (Figure 1). In this case, we observe that the secrets of TRAMP-C1 mouse prostate adenocarcinoma cells induced overexpression of the OPG and RANK-L genes, causing an increase in the OPG/RANK-L ratio, in addition to overexpressing the osteogenic transcription factor Runx2 in osteocytes MLO-Y4 (Figure 5A-D). Given the gene overexpression of Runx2 in these cells, we wanted to check if the osteocalcin protein, a protein associated with bone formation and regulated by Runx222, also underwent changes in these conditions. Similar to Runx2, the expression of osteocalcin increased after stimulation with the secretion of TRAMP-C1 cells in MLO-Y4 osteocytes (Figure 5E).

Figure 5 Soluble factors secreted by prostate tumor cells (TRAMP-C1) modify the expression of bone remodeling genes in osteocytes (MLO-Y4) but not in pre-osteoblasts (MC3T3-E1). The expression of OPG (A, F), RANK-L (B, G), OPG/RANK-L ratio (C, H), Runx2 (D, I) and osteocalcin (E, J) was evaluated by time PCR after stimulating 24 hours MLO-Y4 and MC3T3-E1 with conditioned medium of TRAMP-C1. Data are represented as the mean ± SEM of three independent experiments in triplicate. *p<0.05 vs control
Similar to the data observed with the secretomas of B16 melanoma cells, the conditioned media of TRAMP-C1 cells did not affect the gene expression of the markers previously mentioned in MC3T3-E1 osteoblasts (Figure 5F-J).
DISCUSSION
The formation of a microenvironment that favors the implantation of circulating tumor cells was described by Kapplan. Their results demonstrated the formation of a pre-metastatic niche, in which a series of molecular and cellular changes were observed in the lung prior to the establishment of metastatic melanoma15,23. Other researchers subsequently described a series of sequential events that could involve the formation of a pre-metastatic niche in the liver, suggesting the involvement of exosomes derived from malignant pancreatic lesions as triggers of the process14. In this sense, little has been described about the formation of the pre-metastatic bone niche and the responses of the different bone subpopulations to the stimulation by secretomes of different tumors with high metastatic frequency to bone are unknown. In this article, we show for the first time how the secretome of solid tumors, with high organotropic potential in the formation of bone metastases, modifies the gene expression of genes related to bone metabolism in osteogenic lineage cells, and can be the triggering process in the induction of a favorable microenvironment for the settlement of tumor cells.
It has recently been suggested that primary tumor cells produce soluble tumor factors that trigger immature pre-metastatic niche formation24. Our results confirm that the secretomes of primary tumor cells (such as melanoma and prostate), or breast tumor cells derived from non-bone metastatic processes, mainly modify the balance between OPG and RANKL expression levels. This imbalance would decouple the relationship between osteoblastic bone formation and osteoclastic bone resorption, generating the release of growth factors and cytokines and thus initiating a “previous vicious cycle” of feedback that encourages the formation of future metastatic areas in bone10. In this sense, our results suggest the osteocyte as the cell most susceptible to soluble tumor factors. In our in vitro experimental model, in which we treat both osteocitic cells and mouse osteoblastic cells with conditioned media of murine cells of primary melanoma and prostate tumor, we observe significant changes in the gene expression of the osteocitic line without observing significant changes in the osteoblast line.
Given these observations, we conclude that, although both types of tumors will generate different types of metastases in the future, those derived from melanoma with a more osteolytic character25 and those of preferably osteoblastic prostatic adenocarcinoma, the initial stages, in which alterations occur in bone cell physiology, are common. These observations suggest that the modifications that originate in the pre-metastatic niche do not predispose the specific type of skeletal lesions that will develop in metastatic disease.
Notably, we suggest osteocyte as the main target cell of soluble tumor factors. Since there is no human osteocitic cell model of continuous lineage, we could not corroborate the results obtained by the melanoma and prostate cancer secretomes compared to the breast cancer secretomes in these cells.
The study of the changes induced by the secretome of breast cancer cells on bone cells was limited to the effects on the human osteoblastic continuous line hFOBs. The results obtained suggest that breast cancer cells are capable of affecting osteoblasts by soluble factors and that they mainly modulate the gene expression of RANK-L without the need to maintain cross-communication with these cells, confirming the alteration of the OPG/RANKL axis, as well as the changes induced by the other tumor secretomes studied. Given that osteocytes are the majority cells in bone, with a half-life of 25 years, with a multifunctional character and role of director-coordinator of the bone environment26,27, their modulation, either pharmacological or as a therapeutic target, could be key to avoid pre-metastatic niche formation and bone metastasis. It has been proposed that osteocytes, and not the rest of the cells of the osteogenic lineage, are the main source that the osteoclasts require for their formation and activation in the remodeling of the trabecular bone28,29, and may also be the only ones responsible for initiating the vicious cycle of the bone metastatic process. Few studies have established a clear role of the osteocyte and its relationship with cancer. In this sense, Delgado-Calle et al. showed that the osteocytes could be regulators of the proliferation of myeloma-like cancer cells by direct interaction with them through their cytoplasmic prolongations, which are capable of reaching the periodic and endocortical surfaces, as well as the bone marrow surface30. This direct interaction would lead to activation of the Notch signaling pathway in myeloma cells. This pathway mediates cell-to-cell communication, and participates in the control and activation of cell proliferation and death programs. Its pharmacological inhibition, using an inhibitor of said pathway, prevented the proliferation of myeloma induced by osteocytes30. In addition to regulating and coordinating the rest of cell lines in the bone environment, the osteocytes could secrete factors that reach distant cells, such as, for example, primary tumor cells, and modify them. This communication has been suggested in the case of prostate adenocarcinoma, where osteocitic and osteoblastic cells regulated their osteomimetic properties21. In addition, this communication could modulate key signaling pathways in prostate tumor cells, such as calcium intracellular mediators, cyclic AMP and ERK ½31, and may enhance tumor progression of prostate cancer to bone. All these results, together with our results shown in this paper, suggest that in the crosscommunication between the primary tumor and bone cells, the composition of the factors involved in the secretome may vary. During the evolution of the tumor, soluble tumor factors could reach the bone environment and induce changes in their cells. These cells could also send soluble factors to the primary tumor, which will generate changes in the tumor cells inducing them a greater osteomimetic phenotype.
The secretome of these osteomimetic tumor cells (in the figures presented as pre-treated conditioned media), upon reaching the bone environment again, could affect in the same direction or induce different variations of the changing microenvironment towards the formation of the niche. In this sense, in the proteomic analysis of the secretome of two continuous prostate tumor lines with different origin, DU145 (from brain metastases) and PC3 (from bone metastases) 211 differentially expressed secretion proteins were found, which meant a 37.6% of the total proteins analyzed, indicating that the secretion proteins were considerably different between both cell lines32. Based on these investigations and our results, we propose that tumor secretomes modify the bone environment, the osteocytic line being more sensitive (Figure 6). The results shown open a wide field of study in the knowledge of the communication between the primary tumor cells and the cells of the future metastatic organ, in this case, the bone microenvironment. However, they should deepen their knowledge, due to the limitations of the present study. As previously mentioned, nonspecific effects have been avoided as a result of using cell cultures of different species, and an in vitro cell model based on widely used cell lines has been used as a reference for the study of bone metabolism and physiology, in the case of the MC3T3, MLOY-4 and hFOBs; as well as in the study of cancer, in the case of prostate tumor cells, melanoma or breast. With respect to the breast tumor line (MDAMB-231 HTB-26), one of the limitations of its use in in vitro models is its metastatic origin33, its behavior can be seen as a primary tumor and with it metastatic organotrophic communication .

Figure 6 Tumor secretomes modify the bone environment, the osteocytes being the most sensitive cells to these changes. The soluble factors secreted by prostate and melanoma tumors are those that mainly affect the osteocyte, increasing the gene expression of different factors related to bone remodeling. In turn, the osteocytes secrete factors that modify and induce a greater osteomimetic phenotype in the tumor cells themselve
Thus, the other two tumor lines with bone metastatic organotropism whose origin comes from primary tumors have been studied. However, it would be advisable to confirm the results obtained in this work in an in vivo experimentation model where you can study and confirm the changes in the bone microenvironment prior to the establishment of the metastasis, quantifying in the serum/plasma the altered bone cytokines and determining the temporality of bone and primary tumor communication as well as the level of change in bone tissue. The more detailed knowledge of the molecular changes that involve the formation of the pre-metastatic bone niche, as well as the secretory factors that induce it, could provide new therapeutic targets or action protocols, thus improving its prognosis. These actions would reduce the metastatic skeletal events developed by patients with solid tumors such as prostate or breast tumors, thus increasing their quality of life.
Bibliografía
1 Paget S. The distribution of secondary growths in cancer of the breast. Cancer Metastasis Rev. 1989;8:98-101. [ Links ]
2 Müller A. Homey B, Soto H, Ge N, Catron D, Buchanan ME, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50-6. [ Links ]
3 Zhou, W, Fong MY, Min Y, Somlo G, Liu L, Palomares MR, et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell. 2014; 25:501-15. [ Links ]
4 Lu X, Kang Y. Organotropism of breast cancer metastasis. J Mammary Gland Biol Neoplasia. 2007;12:153-62. [ Links ]
5 Weilbaecher KN, Guise TA, McCauley LK. Cancer to bone: A fatal attraction. Nat Rev Cancer. 2011;11:411-25. [ Links ]
6 Ell B, Kang Y. Snapshot: bone metastasis. Cell. 2012;151:690-690.e1 [ Links ]
7 Guise TA. The vicious cycle of bone metastases. J Musculoskelet Neuronal Interact. 2002;2;570-2. [ Links ]
8 Bagi CM. Targeting of therapeutic agents to bone to treat metastatic cancer. Adv Drug Deliv Rev. 2005;57:995-1010. [ Links ]
9 Guise TA. Molecular mechanisms of osteolytic bone metastases. Cancer. 2000;88:2892-8. [ Links ]
10 Kingsley LA, Fournier PG, Chirgwin JM, Guise TA. Molecular biology of bone metastasis. Mol Cancer Ther. 2007;6:2609-17. [ Links ]
11 Kakonen SM, Mundy GR. Mechanisms of osteolytic bone metastases in breast carcinoma. Cancer. 2003;97:834-9. [ Links ]
12 Yoneda T Cellular and molecular mechanisms of breast and prostate cancer metastasis to bone. Eur J Cancer. 1998;34:240-5. [ Links ]
13 Urwin GH, Percival RC, Harris S, Beneton MN, Williams JL, Kanis JA. Generalised increase in bone resorption in carcinoma of the prostate. Br J Urol. 1985;57:721-3. [ Links ]
14 Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol. 2015;17:816-26. [ Links ]
15 Kaplan R, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, et al. VEGFR-1 positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005; 438:820-7. [ Links ]
16 Kan C, Vargas G, Pape FL, Clézardin P Cancer cell colonisation in the bone microenvironment. Int J Mol Sci. 2016;17(10):E1674. [ Links ]
17 Yu KJ, Li JK, Lee YC, Yu G, Lin SC, Pan T, et al. Cabozantinib-induced osteoblast secretome promotes survival and migration of metastatic prostate cancer cells in bone. Oncotarget. 2017;8(43): 74987-5006. [ Links ]
18 Scheerlinck JP Functional and structural comparison of cytokines in different species. Vet Immunol Immunopathol. 1999;72:39-44. [ Links ]
19 Strober W. Trypan blue exclusion test of cell viability. Curr Protoc Immunol. 2001 May; Appendix 3: Appendix 3B. [ Links ]
20 Gingrich JR, Barrios RJ, Morton RA, Boyce BF, DeMayo FJ, Finegold MJ, et al. Metastatic prostate cancer in a transgenic mouse. Cancer Res. 1996; 56:4096-102. [ Links ]
21 Ardura JA, Gutiérrez-Rojas I, Álvarez-Carrión L, Rodríguez-Ramos MR, Pozuelo JM, Alonso V. The secreted matrix protein mindin increases prostate tumor progression and tumor-bone crosstalk via ERK 1/2 regulation. Carcinogenesis. 2019;40(7):828-39. [ Links ]
22 Alonso V, de Gortázar AR, Ardura JA, Andrade-Zapata I, Alvarez-Arroyo MV, Esbrit P. Parathyroid hormone-related protein (107-139) increases human osteoblastic cell survival by activation of vascular endothelial growth factor receptor-2. J Cell Physiol. 2008;217:717-27. [ Links ]
23 Kaplan RN, Psaila B, Lyden D. Bone marrow cells in the 'pre-metastatic niche': within bone and beyond. Cancer Metastasis Rev. 2007;25:521-9. [ Links ]
24 Liu Y, Cao X. Characteristics and significance of the pre-metastatic niche. Cancer Cell, 2016;30(5):668-81. [ Links ]
25 Mohammad KS, Javelaud D, Fournier PG, Niewolna M, McKenna CR, Peng XH, et al. TGF-β-RI kinase Inhibitor SD-208 reduces the development and progression of melanoma bone metastases. Cancer Res. 2011;71(1):175-84. [ Links ]
26 Atkinson EG, Delgado-Calle J. The emerging role of osteocytes in cancer in bone. JBMR Plus. 2019;3(3):e10186. [ Links ]
27 Bonewald LF. The amazing osteocyte. J Bone Miner Res. 2011;26(2):229-38. [ Links ]
28 Xiong J, Piemontese M, Onal M, Campbell J, Goellner JJ, Dusevich V, et al. Osteocytes, not osteoblasts or lining cells, are the main source of the RANKL required for osteoclast formation in remodeling bone. PLoS One. 2015;10(9):e0138189. [ Links ]
29 Compto, JT, Lee FY. A review of oste-ocyte function and the emerging importance of sclerostin. J Bone Joint Surg Am. 2014;96(19):1659-68. [ Links ]
30 Delgado-Calle J, Anderson J, Cregor MD, Hiasa M, Chirgwin JM, Carlesso N, et al. Bidirectional Notch signaling and os-teocyte-derived factors in the bone marrow microenvironment promote tumor cell proliferation and bone destruction in multiple myeloma. Cancer Res. 2016;76:1089-100. [ Links ]
31 Ardura JA, Gutiérrez Rojas I, Álvarez Ca-rrión L, Friedman PA, Alonso V. Factors secreted by bone cells induce intracellular calcium accumulation and cyclic AMP and activation of ERK 1/2 in prostate cancer cells; evaluation by fluorescence techniques in living cells. Rev Osteoporos Metab Miner. 2018;10 (4):131-8. [ Links ]
32 Kwon OK, Jeon JM, Sung E, Na AY, Kim SJ, Lee S. Comparative secretome profiling and mutant protein identification in metastatic prostate cancer cells by quantitative mass spectrometry-based proteomics. Cancer Genomics Proteomics. 2018;15(4):279-90. [ Links ]
33 Ali R, Samman N, Al Zahrani H, Nehdi A, Rahman S, Khan AL, et al. Isolation and characterization of a new naturally immortalized human breast carcinoma cell line, KAIMRC1. BMC Cancer. 2017; 17(1):803. [ Links ]
Received: July 12, 2019; Accepted: October 07, 2019











 texto em
texto em 



