Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista de Osteoporosis y Metabolismo Mineral
versão On-line ISSN 2173-2345versão impressa ISSN 1889-836X
Rev Osteoporos Metab Miner vol.11 no.2 Madrid Abr./Jun. 2019 Epub 20-Jan-2020
https://dx.doi.org/10.4321/s1889-836x2019000200007
REVIEW
Vitamin D and heart failure. Pathophysiology, prevalence, and prognostic association
1Servicio de Cardiología - Hospital Universitario Dr. Negrín - Las Palmas de Gran Canaria (España)
2Departamento de Ciencias Médico-Quirúrgicas - Universidad de Las Palmas de Gran Canaria - Las Palmas de Gran Canaria (España)
3Unidad Metabólica Ósea - Hospital Insular - Las Palmas de Gran Canaria (España)
INTRODUCTION
Heart failure (HF) is a major public health problem characterized by high mortality, frequent hospitalizations and deterioration in the quality of life, with a prevalence and incidence that is increasing worldwide1,2. Although the prognosis has improved in recent decades thanks to the diagnostic and therapeutic improvement of cardiovascular diseases, the morbidity and mortality of these patients remains high3. All this implies that new objectives and treatment options are still needed.
Vitamin D had traditionally been associated only with bone health, accepting that vitamin D deficiency caused osteomalacia and osteoporosis in adults and rickets in children4,5. However, data obtained in recent years indicate that vitamin D is an important micronutrient for optimal function of many organs and tissues throughout the body, including the cardiovascular system6,7. It has been suggested that vitamin D deficiency may be an important factor both in the genesis of risk factors and cardiovascular disease7 as a prognostic marker in HF. Pathophysiological data indicate that vitamin D deficiency may be very harmful for patients with HF and that vitamin D supplementation can be potentially beneficial, although all this is not without controversy8.
In this paper, we review the evidence that so far supports the link between vitamin D and HF. We analyze the potential mechanisms through which vitamin D could exert, its cardioprotective effects, the potentially deleterious effects of its deficit and break down the main studies on vitamin D supplementation in patients with HF.
PATHOPHYSIOLOGY OF VITAMIN D AND HEART FAILURE
There is no single established route or a single hypothesis that explains the relationship between vitamin D and HF. The vitamin D receptor (RVD) is a nuclear hormonal receptor that mediates the action of calcitriol through genomic and non-genomic pathways9. Cardiomyocytes have RVD, and it is known that calcitriol through RVD also modulates important genes related to cardiovascular health, so they may be influenced by vitamin D10.
Functional RVDs are expressed in the cell nucleus or adjacent to the T tubules of cardiomyocytes, and also of cardiac fibroblasts. Cardiac hypertrophy has been associated with an increase in the expression of these receptors in these cells. Vitamin D has also been attributed an antiproliferative property mediated by the suppression of proto-oncogenes such as c-mic, as well as the natriuretic peptide, acting directly on the growth and differentiation of cardiomyocytes. Mice without RVD (knock-out of the VDR gene) show a greater deposition of collagen in their cardiac structures10.
There are also more complex molecular mechanisms that can explain the relationship between vitamin D and HF. Vitamin D acts on the calcium channels of the cardiomyocytes inducing a rapid intracellular calcium influx11. This intracellular calcium concentration controls long-term growth, proliferation and cell death responses. In addition, by activating the protein C-kinase, it promotes cardiomyocyte relaxation and, therefore, participates in cardiac diastolic function12 and in cardiac systole through activation of adenyl cyclase or cyclic adenosine monophosphate. Dysfunction of any of these pathways can cause systolic and/or diastolic ventricular dysfunction, and, therefore, HF.
Several neuroendocrine systems and inflammatory cytokines are involved in the pathophysiology of vitamin D and HF. These are activated to maintain circulatory homeostasis, but in the long term they contribute to increased systemic resistance and ventricular remodeling, developing and worsening HF. Although the renin-angiotensin aldosterone system (SRAA) and the sympathetic nervous system (SNS) have so far been the most important in HF, recently, both at the diagnostic and therapeutic level, counter-regulatory systems such as natriuretic peptides are also being key. in the diagnostic-therapeutic approach of this syndrome13,14.
It has been shown that vitamin D has an intimate relationship with SRAA. Different studies have shown that there is an inverse correlation between vitamin D levels and the activity of SRAA15-17. The main actions of SRAA include the regulation of vascular tone, volemia, ventricular and vascular remodeling and activation of SNS. The key role of SARS in the pathophysiology of HF and arterial hypertension is well defined.
The cascade of action and pathophysiology of SRAA is as follows: renin is a protein that acts on angiotensinogen, producing angiotensin I, which is transformed into angiotensin II by the action of angiotensin converting enzyme at the pulmonary and vascular level. Angiotensin II is a potent vasoconstrictor hormone of afferent and efferent renal arterioles, and also promotes the activation of the sympathetic nervous system (also key in the pathophysiology of HF). The overactivation of SNS and SRAA contributes to the progressive cardiac remodeling that can lead to HF. This hormone in turn also promotes the release of aldosterone from the adrenal cortex, important in electrolyte and volume balance by retaining sodium and water and releasing potassium and magnesium at the renal level18.
Although one of the main factors that stimulate the release of renin, and therefore SRAA, is the decrease in renal perfusion, experimental studies have shown that after modifying the RVD function in experimental animals with knock-out RVD mice, an increase in the concentration of renin expression with increased mRNA and its protein in the kidney, and plasma angiotensin II, compared to wild-type mice15. Consequently, these knockout mice developed more arterial hypertension, left ventricular hypertrophy and an increase in fluid retention. Injection of 1.25 (OH) 2D achieved marked suppression of renin, which was also achieved with the use of the angiotensin-converting enzyme inhibitor, captopril, or angiotensin II receptor antagonist, losartan; which showed the key pathophysiological role of SRAA15,19, demonstrating that the probable cause of this is overstimulation of SRAA20. The role of angiotensin II in increasing fibrosis and cardiac hypertrophy, increasing vascular tone and therefore blood pressure, as well as increasing sympathetic tone, and direct relationship with the symptoms and progression of heart failure in humans, is also clearly established.
Vitamin D deficiency has been linked to an increase in the production and release of inflammatory cytokines, which has an indirect and direct negative effect on the heart and other organs. Inflammatory cytokines induce apoptosis of cardiomyocytes, hypertrophy, fibrosis, cardiac remodeling and negative ionic alterations such as sodium retention and, therefore, fluid retention21. It also increases catabolic activity and induces cachexia, which contributes to the progression of HF syndrome22. In vitro studies have suggested that vitamin D inhibits inflammatory cytokines such as TNF-α and IL-6, while stimulating anti-inflammatory cytokines such as IL-1023.
RVDs are also present in the parathyroid gland, and calcitriol suppresses the production of parathyroid hormone (PTH) and prevents the proliferation of parathyroid glands24. When there is a vitamin D deficiency, secondary hyperparathyroidism occurs, which also has deleterious cardiovascular and trophic effects on cardiomyocytes. This increase in PTH levels also leads to an increase in blood pressure due to an increase in arterial stiffness and, therefore, and once again, contributes to cardiac remodeling in HF secondary to hypertrophy, apoptosis and fibrosis of the ventricle10,25.
Another pathophysiological mechanism is the influence of vitamin D on the regeneration of the myocardial extracellular matrix, another route by which it can be harmful for cardiac structure and function. Experimental studies with knock-out RVD mice have shown that the absence of vitamin D is associated with an increase in the expression and activity of myocardial matrix metalloproteinases (MMP), which results in myocardial remodeling, increased collagen deposition and greater fibrosis26,27. Vitamin D modulates the regeneration of the extracellular matrix of the myocardium by acting on the expression of both matrix metalloproteinases (MMPs) that hydrolyse extracellular matrix (ECM) proteins and tissue inhibitors of metalloproteinases (TIMP). In knockout RVD mice, the unbalanced expression of MMP/TIMP was characterized by the positive regulation of the MMP2 and MMP-9 negative metalloproteinases of TIMP-1 and TIMP-3. The imbalance between MMP and TIMP promoted the destruction of myocardial tissue and ventricular remodeling; All this is closely related to the complex processes of initiation and progression of diastolic and systolic HF28. It should also be noted that certain inflammatory cytokines such as TNF-alpha are also an important regulator of MMP activity, and can contribute to this pathophysiological pathway29.
Coronary artery disease is an important factor for the development of HF, and vitamin D deficiency has been associated with an increase in arteriosclerosis and calcification of the coronary arteries1,9,27,30. This observation is consistent with the inverse objectified relationship between vitamin D levels and coronary artery calcification6,30,31. It is documented that endothelial cells also express RVD, and that vitamin D increases nitric oxide activity in vitro32, improves vascular endothelial growth factor production33 and reduces endothelial platelet aggregation34. Finally, there is evidence that vitamin D deficiency may be an important regulatory factor of the cardiorenal system. As we have previously emphasized, the cardiovascular and renal system are closely related, so that alterations in the functioning of one can progressively deteriorate the other34.
When there is a progression of cardiorenal syndrome, this also implies neurohormonal activation, mainly of the renin-angiotensin system and the sympathetic nervous system, and of inflammatory systemic mechanisms as described previously. This once again influences fibrosis and ventricular remodeling, hydroelectrolytic abnormalities, and cardiac and renal dysfunction; triggering a negative vicious circuit in response to deterioration of the cardiorenal system, with greater neurohormonal activation and inflammatory cytokines, resulting in greater systemic dysfunction.
In the population with chronic kidney disease (CKD), as in the population with HF, the prevalence of hypovitaminosis D is high, and has also been associated with an increased risk of cardiovascular events34. A reduction in enzyme 1-alpha-hydroxylase activity and the depletion of vitamin D binding proteins to RVD secondary to proteinuria are responsible for patients with CKD having a vitamin D deficiency. Therefore, the close correlation of HF and chronic kidney disease highlights the importance of vitamin D in both pathologies and in the pathophysiology of cardiorenal syndrome.
PREVALENCE OF HYPOVITAMINOSIS D AND HEART FAILURE
Although there is no consensus on the optimal levels of vitamin D, the deficiency of this hormone is defined by most experts as a level of 25-hydroxy-cholecalciferol (25-HCC) below 20 ng/ml4,38-42. To be more specific, according to the consensus uniformly accepted by scientific societies dedicated to bone mineral metabolism, patients are considered to have optimal levels of vitamin D when serum 25-HCC levels are above 30 ng/ml; between 29 and 20 ng/ml it is considered that there is an insufficiency; and below 20 ng/ml the existence of a deficiency is established, which would be severe with 25-HCC figures below 10 ng/ml38,41. Likewise, the theory of the U-shaped relationship between vitamin D levels and any cause of mortality, cardiovascular disease, certain types of cancer, falls and fractures has emerged, and that vitamin D poisoning is observed with serum levels 25-HCC >150 ng/ml43. Clinical practice guidelines recommend that plasma vitamin D levels should not be routinely measured in the general population and should only be measured in patients from populations considered at risk for this hormone deficiency44-47.
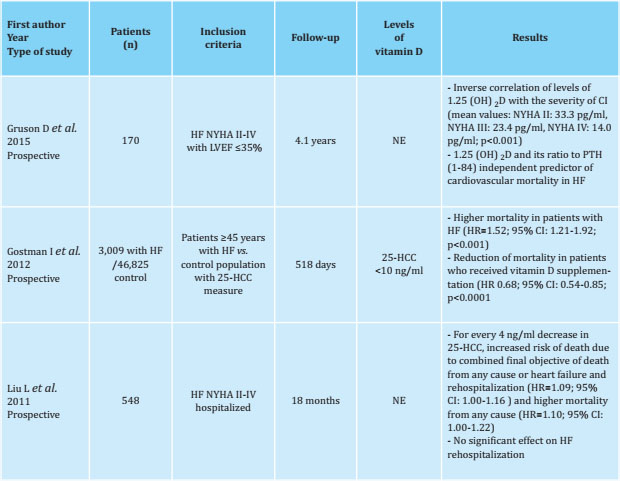
Table 1 Prevalence of vitamin D deficiency in heart failure (HF)
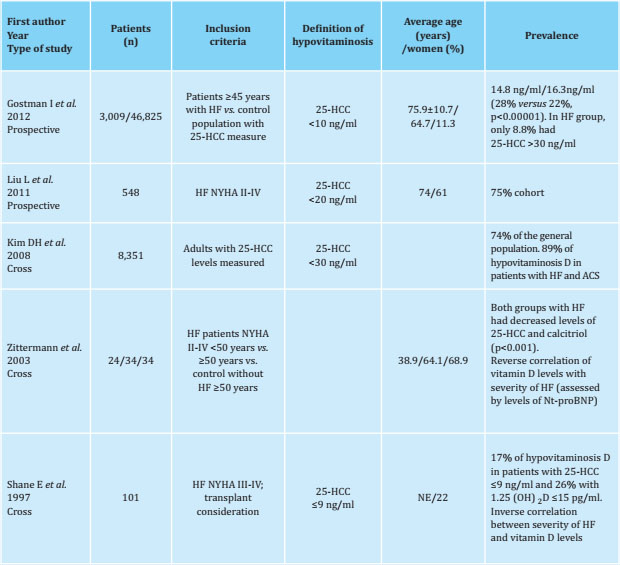
NYHA: New York Heart Association scale; ACS: acute coronary syndrome; Nt-proBNP: N-terminal cerebral natriuretic propeptide; NE: not specified.
In recent years it has been shown that vitamin D deficiency has probably been underestimated and is much more prevalent than had been recognized. A global prevalence of one billion individuals with deficit and insufficient vitamin D levels is estimated42 and it has been described that 40-80% of the adult population has a vitamin D deficit48,49, being of special importance in women in Middle Eastern countries. Numerous risk factors for vitamin D deficiency have been described, such as age, skin hyperpigmentation, hospitalization in institutions, distant latitude of Ecuador, obesity, smoking, nephropathy, hepatopathy or certain drugs such as corticosteroids, phenytoin or phenobarbital49. Of particular relevance is the global obesity epidemic in developed countries, which significantly influences the deficit of vitamin D, given the kidnapping that occurs of this hormone in the adipose tissue48.
It is also true that a vitamin D deficit has been observed in both the young and apparently healthy population50, described in up to approximately 50% of young adults; even in studies carried out in areas with high exposure to sunlight such as the Canary Islands, Israel, Australia, Turkey, India or Lebanon, where 30-50% of children and adults have 25-HCC levels <20 ng/ml51-53.
Likewise, data obtained from the National Health and Nutrition Examination Survey (NHANES) showed a prevalence of hypovitaminosis D of 74% in the general population, significantly increasing the prevalence to 89% when only patients with HF and coronary artery disease were considered. (odds ratio [OR]=3.52; 95% confidence interval [CI]: 1.58-7.84)54. Another study, with similar characteristics in which 4,105 subjects from a general population with at least one determination of vitamin D were included, found that only 36% of this cohort had vitamin D levels within normal range. A higher prevalence of heart failure (90% relative and 9% abso lute) was observed in subjects with vitamin D levels ≤15 ng/ml, and in a follow-up of 1.3±1.2 years of a population over 50 years an incidence of new cases of HF was observed in 2.5% of this cohort. In this study, plasma vitamin D levels was found to have an inverse correlation with HF risk, and the adjusted risk values for HF were 2.01 and 1.3 for values between 16-30 ng/ml. and ≤15 ng/ml, respectively7.
A high prevalence of vitamin D deficiency has also been demonstrated in patients with HF evaluated for heart transplantation, as well as its inverse correlation between serum levels of vitamin D and the severity of HF55. This relationship has also been objectified by other groups using controls without heart failure56 even in younger patients, which suggests that there is an association between HF and vitamin D deficiency that is independent of age.
Therefore, although there is a high prevalence of hypovitaminosis D in the apparently healthy general population, this deficit seems to be more marked in the population with HF (Tables 1, 2 and 3).
RELATIONSHIP BETWEEN VITAMIN D AND HEART FAILURE
HF is a disease with a high socio-sanitary impact, so a special effort has been made in predicting the risk of developing HF and identifying that population at risk in which a more active primary prevention should be emphasized. Therefore, and given the high morbidity and mortality of HF, it is interesting to find prognostic markers in this pathology that can predict mortality. In this regard, attempts have also been made to confirm the association between vitamin D levels and the risk of HF and adverse events through longitudinal studies.
Vitamin D has been associated with the development of HF and as an independent prognostic factor for HF mortality and sudden death in a prospective study of 3,299 Caucasian patients undergoing cardiac catheterization, with a follow-up of 7.7 years on average. When patients with severe hypovitaminosis D (25HCC <10 ng/ml) were compared with patients with optimal levels of vitamin D (25-HCC >30 ng/ml), a hazard ratio (HR) of
2.84 (CI) was obtained. 95%: 1.20-6.74) for death due to HF and 5.05 (95% CI: 2.13-11.97) for sudden death. There was also an inverse correlation between levels of N-terminal cerebral natriuretic propeptide (Nt-proBNP) and serum vitamin D levels, and an inverse association with the NYHA functional class (New York Heart Association)57. These findings have also been subsequently corroborated in a study of 2,312 healthy subjects over 65 years of age, where it was observed that patients with 25-HCC <15 ng/ml had a risk greater than 29% (95%
CI: 5-55% higher ) of mortality from any cause, and that for every 10 ng/ml that decreased 25-HCC increased the relative risk of mortality by 9% (95% CI: 2-17%)20.
To date, data that relate vitamin D levels to the risk of developing HF are discrepant. On the one hand, an unequivocal association of vitamin D levels with the incidence of HF has not been objectified, but on the other, its association with PTH levels20,58,59 has been objectified. Thus, in a study of 6,469 people from a general population free of established cardiovascular disease, with a mean follow-up of 8.4 years, after comparing patients with PTH levels <65 pg/ml and PTH ?65 pg/ml , the latter had a 50% (95% CI: 3-20%) of greater risk of incidence of CI and 5.3 g (95% CI: 2.6-7.9 g) more left ventricular mass determined by RNM58. Likewise, in a cohort of 3,713 men between 60-79 years with and without cardiovascular disease, it was observed that in patients with PTH levels >55.6 pg/ml, there was an increased risk of de novo HF (HR=1, 66; 95% CI: 1.30-2.1)59. These findings were previously demonstrated by Kestenbaum et al. in a study of 2,312 healthy subjects ?65 years of age, in which, after a follow-up of 14 years, they observed that patients with PTH ?65 pg/ml had a greater risk of 30% (95% CI: 6-61%) of incidence of HF20.
This is interesting given that high levels of PTH generally identify patients with low levels of vitamin D, and the relationship between hypovitaminosis D and PTH levels with HF can be confused. In fact, the progressive deterioration of renal function, physical inactivity, as well as the reduction of calcium absorption, are both causes and consequences of hypovitaminosis D, which in turn are associated with an increase in PTH levels. Therefore, in the light of the studies previously exposed, it can be extrapolated that it has been shown that there is an independent association of heart failure risk in patients with low vitamin D levels or elevated PTH levels. This is interesting, as some authors consider that it is the levels of PTH that predict cardiovascular disease60.
Finally, several studies have been published in recent years in which not only a high prevalence of vitamin D deficiency has been observed in patients with HF, but vitamin D has also been linked as a marker of more severe disease and higher rate of adverse events in patients with heart failure. An inverse relationship between 25-HCC levels and B natriuteric peptide (BNP) levels has been observed in patients with HF20,54, as well as ventricular function61, reporting as an independent marker of hospitalization due to HF and mortality20.
However, there is also the theory that vitamin D deficiency in patients with HF occurs because these patients have a worse functional class, are weaker and, therefore, have a more sedentary lifestyle, so they have less exposure to sunlight, which conditions a lower production of vitamin D in the skin and lower concentrations of vitamin D62. This is called into question in different studies in which, after a multivariate analysis with the quantification of physical activity, an association between vitamin D levels and ventricular dysfunction and mortality due to HF is still observed56.
Finally, it is also interesting to comment on the strong association that has been reported among patients with atrial fibrillation and HF (since atrial fibrillation is an important trigger for exacerbation of HF and therapeutic failure) in an observational study in which 180 patients were included. separated into two groups, based on whether they were in sinus rhythm or permanent atrial fibrillation56. In the atrial fibrillation group it was observed that plasma levels of vitamin D were significantly lower (11.05 ng/ml versus 20 ng/ml; p<0.001), PTH levels were significantly higher (76.7 versus 55 pg/ml; p<0.001), and the atrial size was significantly larger (45.03 mm/m2 versus 42.05 mm/m2 ; p<0.01) than in the sinus rhythm group. Vitamin D levels (OR=0.854; 95% CI: 0.805-0.907; p<0.001) and atrial size/body surface area (OR=1.077; 95% CI: 1.003-1.156; p were found to be independent predictors of atrial fibrillation <0.05). In this study, the level of vitamin D was established as a predictive cut-off point for atrial fibrillation at 16.50 ng/ml (76.0% sensitivity and 65.5% specificity, area under curve -AUC- =0.75; 95% CI: 0.67-0.82).
In conclusion, there is experimental and clinical evidence that demonstrates plausible pathophysiological mechanisms and a direct and indirect association between vitamin D with HF and the cardiovascular system. Vitamin D deficiency is very high in patients with HF and could be associated with the prognosis of these patients4,6,8,10,17,19,20,24,30,31,35-37
Bibliografía
1 Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart. 2007; 93(9):1137-46. [ Links ]
2 Sayago-Silva I, García-López F, Segovia-Cubero J. Epidemiology of heart failure in Spain over the last 20 years. Rev Esp Cardiol (Engl Ed). 2013;66 (8):649-56. [ Links ]
3 Ponikowski P, Anker SD, AlHabib KF, Cowie MR, Force TL, Hu S, et al. Heart failure: preventing disease and death worldwide. ESC Hear Fail. 2014;1(1):4-25. [ Links ]
4 Pettifor JM. Nutritional rickets: deficiency of vitamin D, calcium, or both? Am J Clin Nutr. 2004;80:1725S-9. [ Links ]
5 Holick MF. Resurrection of vitamin D and rickets. J Clin Invest. 2006;116(8): 2062-72. [ Links ]
6 Judd SE, Tangpricha V. Vitamin D deficiency and risk for cardiovascular disease. Am J Med Sci. 2009;338(1):40-4. [ Links ]
7 Anderson JL, May HT, Horne BD, Bair TL, Hall NL, Carlquist JF, et al. Relation of vitamin D deficiency to cardiovascular risk factors, disease status, and incident events in a general healthcare population. Am J Cardiol. 2010;106(7):963-8. [ Links ]
8 D’Amore C, Marsico F, Parente A, Paolillo S, De Martino F, Gargiulo P, et al. Vitamin D deficiency and clinical outcome in patients with chronic heart failure: A review. Nutr Metab Cardiovasc Dis. 2017;27(10):837-49. [ Links ]
9 Norman AW. From vitamin D to hormone D: Fundamentals of the vitamin D endocrine system essential for good health. Am J Clin Nutr. 2008;88(2):491s-9s. [ Links ]
10 Camici M, Galetta F, Franzoni F, Carpi A, Zangeneh F. Vitamin D and heart. Intern Emerg Med. 2013;8(Suppl 1):5-9. [ Links ]
11 Simpson RU, Hershey SH, Nibbelink KA. Characterization of heart size and blood pressure in the vitamin D receptor knockout mouse. J Steroid Biochem Mol Biol. 2007;103(3-5):521-4. [ Links ]
12 Green JJ, Robinson DA, Wilson GE, Simpson RU, Westfall MV. Calcitriol modulation of cardiac contractile performance via protein kinase C. J Mol Cell Cardiol. 2006;41(2):350-9. [ Links ]
13 McMurray JJ, Packer M, Desay A, Gong J, Lefkowitz M. Angiotensin–neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2016;291-3. [ Links ]
14 Bayes-Genis A, Morant-Talamante N, Lupón J. Neprilysin and natriuretic peptide regulation in heart failure. Curr Heart Fail Rep. 2016;13(4):151-7. [ Links ]
15 Li YC, Kong J, Wei M, Chen Z, Liu SQ, Cao L. 1,25-Dihydroxyvitamin D3 is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110(2):229-38. [ Links ]
16 Tomaschitz A, Pilz S, Ritz E, Grammer T, Drechsler C, Boehm BO, et al. Independent association between 1,25-dihydroxyvitamin D, 25-hydroxyvitamin D and the renin-angiotensin system. The Ludwigshafen Risk and Cardiovascular Health (LURIC) study. Clin Chim Acta. 2010;411(17-18):1354-60. [ Links ]
17 Forman JP, Williams JS, Fisher NDL. Plasma 25-hydroxyvitamin D and regulation of the renin-angiotensin system in humans. Hypertension. 2010;55(5):1283-8. [ Links ]
18 Peach MJ. Renin-angiotensin system: biochemistry and mechanisms of action. Physiol Rev. 2017;57(2):313-70. [ Links ]
19 Li YC. Vitamin D regulation of the renin-angiotensin system. J Cell Biochem. 2003;88(2):327-31. [ Links ]
20 Kestenbaum B, Katz R, De Boer I, Hoofnagle A, Sarnak MJ, Shlipak MG, et al. Vitamin D, parathyroid hormone, and cardiovascular events among older adults. J Am Coll Cardiol. 2011;58(14): 1433-41. [ Links ]
21 Hedayat M, Mahmoudi MJ, Rose NR, Rezaei N. Proinflammatory cytokines in heart failure: Double-edged swords. Heart Fail Rev. 2010;15(6):543-62. [ Links ]
22 Nozaki N, Yamaguchi S, Shirakabe M, Nakamura H, Tomoike H. Soluble tumor necrosis factor receptors are elevated in relation to severity of congestive heart failure. Jpn Circ J. 1997; 61:657-64. [ Links ]
23 Mora JR, Iwata M, Von Andrian UH. Vitamin effects on the immune system: Vitamins A and D take centre stage. Nat Rev Immunol. 2008;8(9):685-98. [ Links ]
24 DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80(6 Suppl):1689S-96S. [ Links ]
25 Saleh FN, Schirmer H, Sundsfjord J, Jorde R. Parathyroid hormone and left ventricular hypertrophy. Eur Heart J. 2003;2054-60. [ Links ]
26 Gunja-Smith Z, Morales AR, Romanelli R, Woessner JF. Remodeling of human myocardial collagen in idiopathic dilated cardiomyopathy. Role of metalloproteinases and pyridinoline cross-links. Am J Pathol. 1996;148(5):1639-48. [ Links ]
27 Li YY, Feng YQ, Kadokami T, McTiernan CF, Draviam R, Watkins SC, et al. Myocardial extracellular matrix remodeling in transgenic mice overexpressing tumor necrosis factor alpha can be modulated by anti-tumor necrosis factor alpha therapy. Proc Natl Acad Sci. 2002;97(23):12746-51. [ Links ]
28 Weber KT, Weglicki WB, Simpson RU. Macro- and micronutrient dyshomeostasis in the adverse structural remodelling of myocardium. Cardiovasc Res. 2009;81(3):500-8. [ Links ]
29 Li YY, Kadokami T, Wang P, McTiernan CF, Feldman AM. MMP inhibition modulates TNF-α transgenic mouse phenotype early in the development of heart failure. Am J Physiol Circ Physiol. 2015;282(3):H983-9. [ Links ]
30 Akin F, Ayça B, Köse N, Duran M, Sarı M, Uysal OK, et al. Serum vitamin D levels are independently associated with severity of coronary artery disease. J Investig Med. 2016;60(6):869-73. [ Links ]
31 de Boer IH, Kestenbaum B, Shoben AB, Michos ED, Sarnak MJ, Siscovick DS. 25-hydroxyvitamin D levels inversely associate with risk for developing coronary artery calcification. J Am Soc Nephrol. 2009;20(8):1805-12. [ Links ]
32 Molinari C, Uberti F, Grossini E, Carda S, Invernizzi M, Cisari C. 1α,25-dihydroxycholecalciferol induces nitric oxide production in cultured endothelial cells. Cell Physiol Biochem. 2011; 27(6):661-8. [ Links ]
33 Grundmann M, Haidar M, Placzko S, Niendorf R, Darashchonak N, Hubel CA, et al. Vitamin D improves the angiogenic properties of endothelial progenitor cells. Am J Physiol Physiol. 2012;303(9):954-62. [ Links ]
34 Stach K, Kälsch AI, Nguyen XD, Elmas E, Kralev S, Lang S, et al. 1α,25-dihydroxyvitamin D 3 attenuates platelet activation and the expression of VCAM-1 and MT1-MMP in human endothelial cells. Cardiology. 2011;118(2):107-15. [ Links ]
35 Al Mheid I, Quyyumi AA. Vitamin D and cardiovascular disease: controversy unresolved. J Am Coll Cardiol. 2017;70(1):89-100. [ Links ]
36 Rostand SG, Drüeke TB. Parathyroid hormone, vitamin D, and cardiovascular disease in chronic renal failure. Kidney Int. 1999;56(2):383-92. [ Links ]
37 Levin A, Bakris GL, Molitch M,Smulders M, Tian J, Williams LA, et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: Results of the study to evaluate early kidney disease. Kidney Int. 2007;71(1):31-8. [ Links ]
38 Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84(1):18-28. [ Links ]
39 Giustina A, Adler RA, Binkley N, Bouillon R, Ebeling PR, Lazaretti-Castro M, et al. Controversies in vitamin D: summary statement from an international conference. J Clin Endocrinol Metab. 2019;104(2):234-40. [ Links ]
40 Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911-30. [ Links ]
41 Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporos Int. 2005;16(7):713-6. [ Links ]
42 Holick MF. High Prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81(3):353-73. [ Links ]
43 Ross AC, Manson JAE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53-8. [ Links ]
44 Glendenning P, Inderjeeth CA, Holick M. Measuring vitamin D. Clin Biochem. 2012;38(12):901-6. [ Links ]
45 Glendenning P, Inderjeeth CA. Vitamin D: Methods of 25 hydroxyvitamin D analysis, targeting at risk populations and selecting thresholds of treatment. Clin Biochem. 2012;45(12):901-6. [ Links ]
46 Kennel K, Drake MT, Hurley DL. Vitamin D deficiency in adults: when to test and how to treat. Mayo Clin Proc. 2010;85(8):752-8. [ Links ]
47 Holick MF. NIH public vitamin D status: measurement, interpretation and clinical application. Ann Epidemiol. 2009;19(2):73-8. [ Links ]
48 Palacios C, Gonzalez L. Is vitamin D deficiency a major global public health problem? J Steroid Biochem Mol Biol. 2014;144:138-45. [ Links ]
49 Holick MF, Chen TC. Vitamin D deficiency: a worldwide health problem. Am J Clin Nutr. 2008;87:1080-6. [ Links ]
50 Groba Marco M, Mirallave Pescador A, González Rodríguez E, García Santana S, González Padilla E, Santana P S, et al. Factores relacionados con insuficiencia de vitamina D en estudiantes de Medicina de Gran Canaria. Rev Osteoporos Metab Miner. 2010;2:11-8. [ Links ]
51 Marwaha RK, Tandon N, Reddy DRHK, Aggarwal R, Singh R, Sawhney RC, et al. Vitamin D and bone mineral density status of healthy schoolchildren in northern India. Am J Clin Nutr. 2005; 82(2):477-82. [ Links ]
52 Fuleihan GE-H, Nabulsi M, Choucair M, Salamoun M, Shahine CH, Kizirian A, et al. Hypovitaminosis D in healthy schoolchildren. Pediatrics. 2004;107 (4):e53-e53. [ Links ]
53 McGrath J, Kimlin M, Saha S, Eyles D, Parisi A. Vitamin D insufficiency in south-east Queensland. Med J Austr. 2001;174; 150-151. [ Links ]
54 Kim DH, Sabour S, Sagar UN, Adams S, Whellan DJ. Prevalence of hypovitaminosis D in cardiovascular diseases (from the National Health and Nutrition Examination Survey 2001 to 2004). Am J Cardiol. 2008;102(11):1540-4. [ Links ]
55 Shane E, Mancini D, Aaronson K, Silverberg SJ, Seibel MJ, Addesso V, et al. Bone mass, vitamin D deficiency, and hyperparathyroidism in congestive heart failure. Am J Med. 1997;103(3):197-207. [ Links ]
56 Zittermann A, Schulze Schleithoff S, Tenderich G, Berthold HK, Körfer R, Stehle P. Low vitamin D status: A contributing factor in the pathogenesis of congestive heart failure? J Am Coll Cardiol. 2003;41(1):105-12. [ Links ]
57 Pilz S, März W, Wellnitz B, Seelhorst U, Fahrleitner-Pammer A, Dimai HP, et al. Association of vitamin D deficiency with heart failure and sudden cardiac death in a large cross-sectional study of patients referred for coronary angiography. J Clin Endocrinol Metab. 2008;93(10):3927-35. [ Links ]
58 Bansal N, Zelnick L, Robinson-Cohen C, Hoofnagle AN, Ix JH, Lima JA, et al. Serum parathyroid hormone and 25hydroxyvitamin D concentrations and risk of incident heart failure: The Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc. 2014;3(6):1-15. [ Links ]
59 Wannamethee GS, Welsh PW, Papacosta OP, Lennon L, Whincup PH, Sattar N. Elevated parathyroid hormone, but not vitamin D deficiency, is associated with increased risk of heart failure in older men with and without cardiovascular disease. Circ Hear Fail. 2014;7(5):732-9. [ Links ]
60 Kamycheva E, Sundsfjord J, Jorde R. Serum parathyroid hormone levels predict coronary heart disease: The Tromso Study. Eur J Prev Cardiol. 2004;11(1):69-74. [ Links ]
61 Zittermann A, Ernst JB. Calciotropic and phosphaturic hormones in heart failure. Nutr Metab Cardiovasc Dis. 2016;26(11):971-9. [ Links ]
62 Zittermann A, Schleithoff SS, Koerfer R. Vitamin D insufficiency in congestive heart failure: Why and what to do about it? Heart Fail Rev. 2006;11(1):25-33. [ Links ]











 texto em
texto em