My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista de Osteoporosis y Metabolismo Mineral
On-line version ISSN 2173-2345Print version ISSN 1889-836X
Rev Osteoporos Metab Miner vol.12 n.1 Madrid Jan./Mar. 2020 Epub June 15, 2020
https://dx.doi.org/10.4321/s1889-836x2020000100004
ORIGINAL ARTICLES
Effects of bazedoxifene treatment on the bone quality of ovariectomized rats
1Biochemical Research. Institute for Medical Research. Jimenez Diaz Foundation. Madrid (Spain).
2Hospital Support Team. Palliative Care. Mostoles University Hospital. Mostoles. Madrid (Spain).
3Department of Physiology. Faculty of Pharmacy. Complutense University of Madrid. Madrid (Spain).
Objetive:
Bazedoxifene is a 3rd generation SERM with agonistic effects on the bones, uterus and breast tissue. Our goal has been to study the effects of bazedoxifene on bone quality of an experimental group of ovariectomized rats.
Material and methods:
3 groups of 15 6-month-old Wistar female rats were used: a control group, a group of untreated ovariectomized rats and a group of ovariectomized rats treated with bazedoxifene (0.33 mg/kg/day). After 8 months we studied the lumbar and femur bone densitometry, the microtomographic parameters, the biochemical markers for bone remodelling and the bone biomechanical parameters.
Results:
The ovariectomy depleted the femur and lumbar bone density. After receiving bazedoxifene, the lumbar bone density showed partial healing. Bone remodelling increased recovering bazedoxifene formation levels. Bazedoxifene promoted the recovery of the bone volume fraction (BV/TV), the bone surface density (BS/BV), the trabecular number (Tb.N), the trabecular spacing (Tb.Sp), the trabecular pattern factor (Tb.Pf) and the structural model index (SMI). The cortical surface increased after the ovariectomy and returned to normal levels with the administration of bazedoxifene. The maximum deformation showed before the ovariectomy was also restored, partially cushioning the ovariectomized rats’ weight gain.
Conclusions:
Our study has shown bazedoxifene positive results on bone quality. This specific drug could be particularly suitable for young postmenopausal women suffering or at risk of suffering osteoporosis.
Key words: Bazedoxifene; bone mineral density; bone remodelling; microtomography; biomechanics; endometrial safety
INTRODUCTION
Selective estrogen-receptor modulators (SERMs) are synthetic, nonsteroidal agents with estrogenic agonist-antagonist activity in different target tissues1. Their estrogenic responses are mediated by estrogen receptors (α and β). SERMs may present agonistic or antagonistic behavior depending on the tissue type2,3. In general, SERMs exhibit agonist activity in the liver, the digestive tube, the skeleton and the heart, but antagonist activity in the breast. In the uterus some SERMs manifest agonist activity while others show an antagonist behavior1. Several co-regulatory proteins modify the behavior of the SERMs on gene expression and contribute to their tissue-selective pharmacology.
Tamoxifen is a SERM used as a mammary antiestrogen for preventing and treating breast cancer with estrogen agonistic activity in the uterus. Raloxifene has been used for the prevention and treatment of osteoporosis and prevents breast cancer but presents some estrogenic activity4. Bazedoxifene is a 3rd generation SERM with agonistic effects on the bone and additional positive effects on lipids, the uterus and the breast tissue5,6.
Due to its estrogen agonistic activity on the bone, raloxifene and bazedoxifene are used to treat osteoporosis. Bazedoxifene has the advantage of a greater endometrial safety, therefore it is as well widely used in combination with conjugated equine estrogens for the treatment of endometriosis7-9.
Our study focuses on the effects produced by bazedoxifene on the bone. However, we find it interesting to point out that bazedoxifene has also been identified as an effective therapeutical agent against human colorectal cancer10, breast cancer11, gastrointestinal cancer12 and gastric adenocarcinoma13.
Regarding the effects on the bone, Keating et al.14 found that bazedoxifene reduced the rates of new vertebr al fractures in patients affected by osteoporosis, as well as the rates of non-vertebral fractures in high-risk patients. Moreover, it is a very well-tolerated drug, without adverse effects on the endometrium or breast tissue7.
The purpose of our research was to study the effects of bazedoxifene on the bone quality in detail, using an experimental group of ovariectomized rats and longterm treatment (8 months). We examined the lumbar and femoral bone densitometry, the microtomographic trabecular and cortical parameters, the biochemical markers reflecting bone formation and bone resorption and the bone biomechanical parameters.
Material and methods:
45 6-month-old Wistar female rats from the Jimenez Diaz Foundation animal facility were used. These rats were kept at a constant temperature of 22ºC, observing 12-hour light-dark cycles and with free access to food and drink. The food was a complete diet for rats and mice (Panlab®, Barcelona, España). The average weight of the rats at the beginning of the study was 333.6±32 g (mean ± standard deviation).
The rats were randomly divided in 3 groups:
SHAM group (n=15): the ovariectomy was simulated;
OVX group (n=15): ovariectomized rats;
OVX + BZD group (n=15): ovariectomized rats, administered 0.33 mg/kg/day of bazedoxifene using a feeding tube for 8 months.
The treatment started the day after the ovariectomy had been performed and continued during the following 8 months. Every single treatment was administered according to the EU directives on the protection of animals used for scientific purposes and were approved by the ethics committee of the Institute for Health Research of the Jimenez Diaz Foundation.
The bazedoxifene drug was Conbriza® (Pfizer), donated by Pfizer Laboratories. The dosage was calculated based on the recommended treatment for osteoporosis in humans, 20 mg/day taken orally, therefore the dose of bazedoxifene we used on our rats was 0.33 mg/kg/day through a feeding tube and 0.3 ml of water for each animal.
For surgery, the rats were anaesthetized via intramuscular injections of 0.7 ml of a 1:2 mixture of 2 g/ml of xylazine hydrochloride (Rompun®) and 50 mg/ml of ketamine (Ketolar®). Once anesthetized, all four limbs were immobilized and the area to be operated on was clipped. The animals were in the supine position, leaning on their backs. The bilateral ovariectomy surgery was carried out through an abdominal incision. To remove the ovaries, the uterine horns were identified, one end attached to the ovary and the other to the uterus. When ties were established on either side of the ovary, we proceeded to section and remove them. Once this process finished, the incision was stitched. After 8 months of treatment, the rats were weighed and sacrificed via exsanguination by a heart perfusion under anaesthesia with Isoflurane (Forane®). Through this perfusion, we obtained the blood samples that would be centrifuged at 3,000 r.p.m. for 15 minutes to obtain the serum. This serum was divided into aliquots and frozen at -80ºC, up to the moment the bone remodelling parameters were to be determined.
After extracting their blood, the rats were frozen at -20ºC until bone mineral density measurement had to be taken. The day before such procedure, the rats were introduced in a fridge at -20ºC in order to thaw. Then their right and left femur were amputated using scalpel and tweezer. Once the femurs were extracted and cleaned, we performed a bone mineral densitometry on the left femur and spine at L2, L3 and L4 levels.
Bone densitometry
We proceeded to the determination of the bone mineral densitometry (BMD) of the left femur and the spine at L2, L3 and L4 levels, undergoing a dual-energy X-Ray densitometry (DXA). We used a machine called Piximus (Hologic®, QDR-1000 TM), a specific densitometer for animal and small samples.
The BMD scanning was carried out on the femur entirely and on the whole three vertebrae (L2, L3 and L4), and the results were expressed as the average of the obtained values. The inter- and intra-assay coefficients of variability were <0.53% and <1.2% respectively.
After taking this measurement, the femurs were wrapped in gauze soaked in physiological saline solution and kept frozen at -20ºC until the computerized microtomography was carried out. The right femurs were kept in the same way for biomechanical testing. In these circumstances, the mechanical properties of the bone were not found to significantly change for at least 7 or 8 months. Likewise, no variations have been observed after samples go through up to 5 short freezing-thawing periods15.
Biochemical markers of bone remodeling
Blood samples were thawed to determine biochemical markers of bone remodeling.
Biochemical markers of bone formation:
Osteocalcin (BGP): a specific commercial colorimetric immunoassay (ELISA) was used for the determination of osteocalcin levels in rats (Rat-MID™ Osteocalcin, IDS, UK). The sensitivity of the assay was 50 ng/ml, and the inter- and intra-assay coefficients of variability were <5.0% and <6.6%, respectively.
-
Procollagen I amino-terminal propeptide (PINP): a specific commercial enzyme immunoassay (ELISA) was used to determine concentrations of PINP in rats (Rat/Mouse PINP, IDS, UK). The sensitivity of the method was 0.7 ng/ml, and the inter- and intra-assay coefficients of variability <5% and <8.2%, respectively.
Biochemical marker of bone resorption:
Type I collagen carboxy-terminal telopeptide (CTX): a rat-specific ELISA (RatLaps CTX-I ELISA, IDS, UK) was used. The sensitivity of the assay was 2.0 ng/ml and the inter- and intra-assay coefficients of variability of this method were <5.6% and <10.5% respectively.
Microtomography
The left femurs of the rats were sent to the University of Oviedo to study the bone microarchitecture from the computerized microtomography (micro-CT) images got from the bone samples. This analysis was performed in the distal metaphysis of the femur and in a cortical bone ring of its diaphysis.
All samples were scanned on a SkyScan 1174 desktop X-Ray microtomograph (Bruker, Kontich, Belgium). The samples were placed with the long axis perpendicular both to the base of the sample holder and to the X-Ray source. The images were obtained under the following conditions: voltage of the X-Ray source: 50 KV; X-Ray source intensity: 800 μA; use of 1mm aluminum filter; resolution: 17.1 μm; sample rotation step: 0.4°; total rotation: 180°; frame averaging: 2; exposure time: 11,000 ms; approximate scanning time per sample: 3 hours and 50 minutes. 930 tomograms in TIFF format were obtained from each sample.
The flat-field correction was carried out at the beginning of every scan. The tomograms obtained from scanning the samples were reconstructed using the Feldkamp algorithm, modified in the NRecon application, version (Bruker microCT, Kontich, Belgium). The optimal parameters selected were: ring artefact reduction: 8; beam hardening correction: 30´; smoothing: 1.
The scanning and reconstruction parameters used were the same for all samples. After the reconstruction, two different volumes of interest (VOI) were selected using the CTAn application, version 1.14.4.1, (Bruker, Kontich, Belgium) in which to determine the microstructural properties and bone mineral density. In the case of the trabecular bone, a VOI was selected starting at 1 mm from the growth cartilage of the distal metaphysis of the femur (taken as reference section) and occupying 3.4 mm in the proximal direction (a total of 200 images), excluding the cortical bone to be analyzed. For cortical bone analysis, the growth cartilage of the distal metaphysis is again taken as a reference, starting the VOI at 14 mm from it and covering mm (150 images). The structural analysis of the VOI is carried out with the software provided with the equipment (CTAn version 1.14.4.1). Once the results of the microstructural parameters were obtained, the CTVol 2.2.3.0 program (Bruker, Kontich, Belgium) was used to visualize the three-dimensional models created with CTAn using the Marching cubes 33 algorithm.
For the trabecular bone, standard cancellous bone morphometric parameters were determined by a 3D analysis of the trabeculae.
The parameters studied for the trabecular bone are detailed below.
Surface and volume relationships:
The bone volume fraction (BV/TV) perfectly reflects the bone loss or gain in the different groups. It is obtained from the basic morphometric indexes, bone volume (BV) and total volume of interest (TV). It is commonly expressed as a percentage. The total area of the trabecular bone (BS) is measured by triangulating the surface of the object. Its relationship with the volume of interest analyzed is known as bone surface density (BS/TV). It is expressed in mm-1, as it is the quotient between an area unit and a volume unit. The bone specific surface (BS/BV) expresses the relationship between the total area of the trabecular bone with the volume occupied only by mineralized bone. Like the previous variable, it is also expressed in mm-1.
Direct metric indices:
The trabecular thickness (Tb.Th) is calculated following a method that occupies with spheres the structure analyzed by distance transformation. It is usually expressed in mm or μm. The trabecular separation (Tb.Sp) is calculated in the same way, but this time occupying the medullary cavities. It is expressed in mm or μm. The trabecular number (Tb.N) means the number of times trabeculae are traversed by an arbitrary path through the volume of interest per unit length. The method is to launch a line through the region of interest and count how many times it crosses trabeculae. It is expressed in mm-1.
Direct non-metric indices:
The trabecular pattern factor (Tb.Pf) quantitatively describes trabecular connectivity. It is an inverse connectivity index (the higher the Tb.Pf value, the less connected the trabeculae are) based on the calculation of a relative convexity or concavity index of the total bone surface, in which the concavity of the trabecular surfaces implies connectivity, while convexity indicates disconnected and isolated structures. The higher the Tb.Pf value, the worse connectivity the trabecular network shows, which implies a decrease in mechanical resistance. It is expressed in mm-116. The structural model index (SMI) shows the relative prevalence of plate-like or rod-like trabeculae, indicating more presence of plates the closer its value get to zero17. It is defined in a range of values from 0 to 3, where 0 is an ideal plate-shaped structure and 3 is a cylinder. The degree of anisotropy (DA) is a measure of the symmetry of the object or the presence/absence of structures aligned in a certain direction. It is a dimensionless variable. Zero is total isotropy and 1 is total anisotropy. The different variables were directly measured using methods described in the bibliography18,19.
Two different analyses were carried out in the cortical region. The first one (endosteum-periosteum separation) allowed us to calculate total volume, bone volume and medullary volume. In the second one we report the porosity of the cortical bone.
Endosteum-periosteum separation: the total volume of the cross section inside the periosteum (VIP) is the mean value of the volume occupied by bone and bone marrow in the analyzed cross sections. It is expressed in mm3. A low VIP value indicates that there is less bone formation and more resorption, and the other way round if we find a high value. Cortical bone volume (Ct.BV) is the mean value of the volume occupied by bone in the analyzed cross sections. It is expressed in mm3. The medullary volume (Md.V) is the mean value of the volume occupied by the bone marrow in the analyzed cross sections. It is expressed in mm3. This value indicates the opposite of VIP.
Porosity parameters studied: cortical bone volume excluding pores (Ct.BV); the ratio between the cortical surface and the volume of the cortical bone without pores (Ct.BS/BV); and the porosity of the cortical bone (Ct.B.Po).
Biomechanics
The right femurs of the rats remained frozen at -80ºC and were thawed prior to the mechanical test for proper preparation. The test was carried out on a universal testing machine. A 3-point bending test was set up, with a spacing of 17.6 mm and an indenter diameter of 5.6 mm. The force was applied perpendicularly to the axis of the bone, in the region of the diaphysis, with an application speed of 10 mm/min (0.17 mm/s). We obtained a load-displacement curve for each sample and we proceeded to calculate the diameter of the diaphysis from the average of 6 different measurements, to minimize the effect of variability.
Analyzed biomechanical parameters:
From the curve resulting from each experiment, different parameters indicating the mechanical characteristics of the samples have been determined20: maximum bending force at the time of mechanical failure; displacement at the time of mechanical failure; extrinsic stiffness; breaking energy; maximum tension; maximum deformation; and Young's modulus.
Results:
Figure 1 shows the results obtained in the femur bone mineral density (FBMD) and lumbar bone mineral density (LBMD) of the rats studied. Ovariectomy produced a significant decrease in bone density in the femur and spine. Bazedoxifene treatment partially recovered lumbar density, but not femur density.
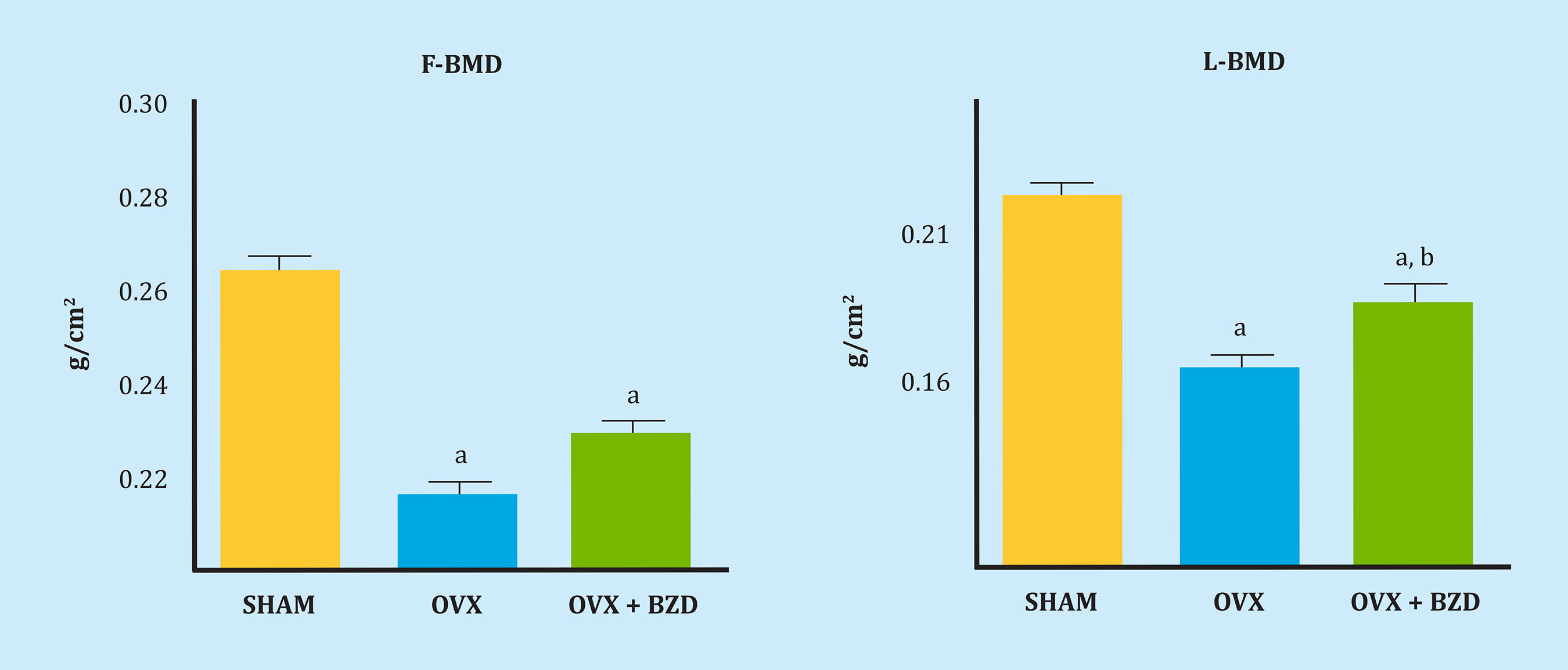
F-BMD. a: OVX vs SHAM, p<0.01; OVX+BZD vs SHAM, p<0.01; L-BMD. a: OVX vs SHAM, p<0.01; OVX+BZD vs SHAM, p<0.05, b: OVX+BZD vs OVX, p<0.05.
Figure 1. Femoral bone mineral density (F-BMD) and lumbar bone mineral density (L-BMD) in the 3 groups of rats: SHAM (control), ovariectomized (OVX) and ovariectomized remodel treated with bazedoxifene (OVX + BZD)
Figure 2 shows the levels of the biochemical markers of bone remodeling in the groups of rats studied. As expected, markers of bone formation and resorption (BGP, PINP, and CTX) experienced a significant increase after ovariectomy. Bazedoxifene treatment recovered the basal levels of BGP and PINP, without significant variations in CTX levels.

BGP. a: OVX vs SHAM, p<0.01, b: OVX+BZD vs OVX, p<0.01; PINP. a: OVX vs SHAM, p<0.01, b: OVX+BZD vs OVX: p<0.01; CTX. a: OVX vs SHAM p<0.01; OVX+BZD vs SHAM p<0.01.
Figure 2. Biochemical markers of bone remodelling: osteocalcin (BGP), aminterminal procollagen I propeptide (PINP) and carboxyterminal collagen I telopeptide (CTX) in the 3 groups of rats: SHAM (control), ovariectomized (OVX) and ovariectomized treated with bazedoxifene (OVX + BZD)
Figure 3 shows a series of studied quantitative microstructural parameter. Bone volume fraction (BV/TV) and bone surface density (BS/TV) decreased after the ovariectomy, partially recovering after the treatment with bazedoxifene. Bazedoxifene also partially recovered the increase in the trabecular separation (Tb.Sp) produced by the ovariectomy, as well as the decrease in the trabecular number (Tb.N), without acting on trabecular thickness (Tb.Th).
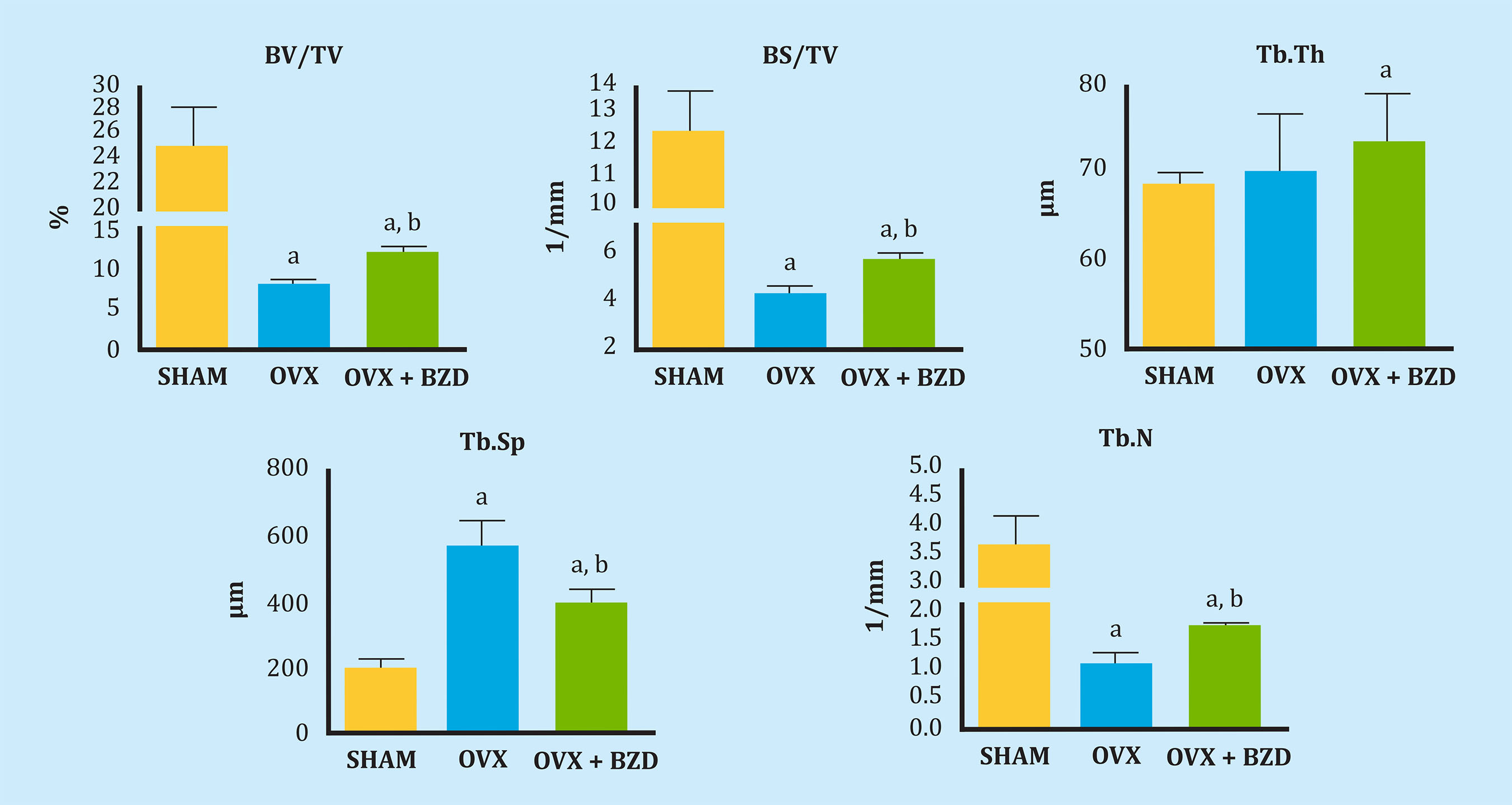
BV/TV. a: OVX vs SHAM p<0.01; OVX+BZD vs SHAM, p<0.05, b: OVX+BZD vs OVX, p<0.05; BS/TV. a: OVX vs SHAM p<0.01; OVX+BZD vs SHAM, p<0.05, b: OVX+BZD vs OVX, p<0.05; Tb.Th. a: OVX+BZD vs SHAM, p<0.05; Tb.Sp. a: OVX vs SHAM p<0.01; OVX+BZD vs SHAM, p<0.05, b: OVX+BZD vs OVX, p<0.05; Tb.N. a: OVX vs SHAM p<0.01; OVX+BZD vs SHAM, p<0.05, b: OVX+BZD vs OVX, p<0.05.
Figure 3. Bone volume fraction (BV/TV), bone surface density (BS/TV), trabecular thickness (Tb.Th), trabecular spacing (Tb.Sp) and trabecular number (Tb.N) in the 3 groups of rats: SHAM (control), ovariectomized (OVX) and ovariectomized treated with bazedoxifene (OVX + BZD)
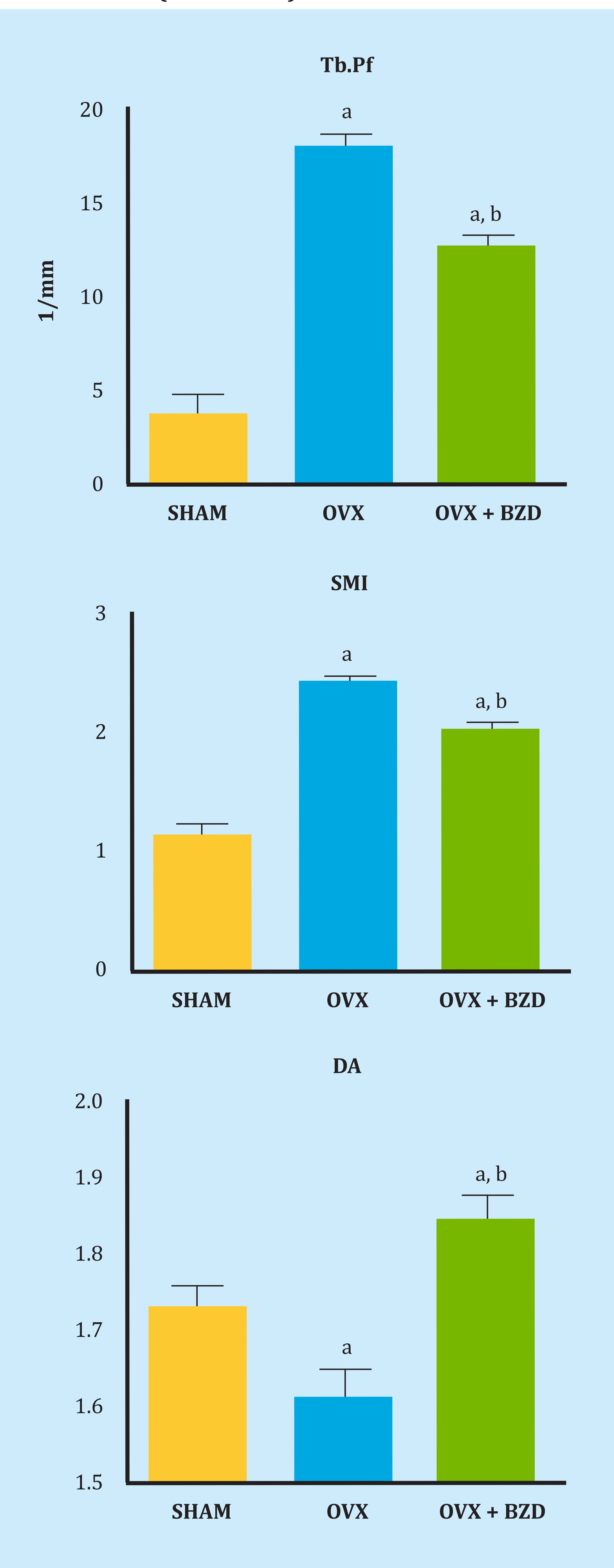
Figure 4 shows the non-metric variables Tb.Pf and SMI and the quantitative variables Conn.Dn and DA in the groups of the studied rats. The trabecular pattern factor Tb.Pf increased significantly in the ovariectomized rats, indicating a significant loss of trabecular connectivity after the ovariectomy. Bazedoxifene treatment partially corrected this loss. The ovariectomy also significantly increased the structural model index SMI, indicating a prevalence of rod-shaped trabeculae, compared to control rats, with a prevalence of plate-shaped trabeculae. Bazedoxifene treatment also partially corrected this variation. The degree of anisotropy decreased significantly after the ovariectomy, increasing after treatment with bazedoxifene to values higher than that of the control rats.

Tb.Pf. a: OVX vs SHAM, p<0.01; OVX+BZD vs SHAM, p<0.05, b: OVX+BZD vs OVX, p<0.05; SMI. a: OVX vs SHAM, p<0.01; OVX+BZD vs SHAM, p<0.05, b: OVX+BZD vs OVX, p<0.0; DA. a: OVX vs SHAM, p<0.05; OVX+BZD vs SHAM, p<0.01, b: OVX+BZD vs OVX, p<0.01.
Figure 4. Trabecular pattern factor (Tb.Pf), structural model index (SMI) and degree of anisotropy (DA). In the 3 groups of SHAM rats (control), ovariectomized (OVX) and ovariectomized rats treated with bazedoxifene (OVX + BZD)
Figure 5 shows the results of bone volume + bone marrow (VIP), cortical bone (Ct.BV) and medullary volume (Md.V) in the cortical. Bone + marrow volume did not seem to have varied significantly after the ovariectomy, but it was lower in the ovariectomized rats treated with bazedoxifene, suggesting an unresolved ovarian failure influenced by this drug. Cortical bone volume (Ct.BV) decreased significantly after the ovariectomy, with bazedoxifene not exerting a positive action. Medullary volume (Md.V) increased after the ovariectomy, remaining constant after bazedoxifene treatment.
CtBv. a: OVX vs SHAM, p<0.05; OVX+BZD vs SHAM, p<0.05; Md.V. a: OVX vs SHAM, p<0.01.
Figure 5. Bone volume + bone marrow (VIP), cortical bone (Ct.BV) and medullary volume (Md.V) in the cortical in the 3 groups of rats: SHAM (control), ovariectomized (OVX) and ovariectomized rats treated with bazedoxifene (OVX + BZD)
Cortical bone volume decreased after the ovariectomy (p<0.05), with bazedoxifene treatment not producing any effects.. The relative cortical surface increased after the ovariectomy (p<0.05), normalizing after treatment with bazedoxifene. The porosity (Ct.B.Po) decreased significantly after the ovariectomy (p<0.001), with bazedoxifene treatment not producing variations.
Maximum displacement, stiffness, break work, maximum tension, and Young's modulus did not vary with the ovariectomy or the break work. The maximum bending force at the time of mechanical failure decreased with the ovariectomy (p<0.05), as expected, with no effect from bazedoxifene. The maximum deformation before rupture decreased with the ovariectomy (p<0.05), recovering with bazedoxifene treatment.
Regarding the weights of the rats, at the end of the experiment the SHAM group weighed 380±25 g, the OVX group 475±30 g (OVX vs SHAM, p<0.01) and the group treated with bazedoxifene 425±15 g (BZD vs SHAM, p<0.05; BZD vs OVX, p<0.05). The ovariectomy made the rats gain weight and the treatment with bazedoxifene partially cushioned this gain.
DISCUSSION
According to our results, bazedoxifene treatment partially recovered lumbar bone density, but not femur bone density.
Coinciding with this, Barrionuevo et al.21 conducted a study including 107 clinical trials in which it could be concluded that there was a significant reduction in vertebral fractures with bazedoxifene. Similarly, Jin et al.22, studying 41 articles from clinical trials from 2015 to 2019, concluded that bazedoxifene prevents vertebral fractures. Peng et al.23 conducting a systematic review of studies carried out over 3 and 7 years, and Palacios et al.24, in a study carried out over 7 years, observed that the incidence of new vertebral fractures was lower in women treated with bazedoxifene than in the placebo group.
Regarding the biochemical markers of bone remodeling, our results show a decrease in the same in BGP and PINP levels after treatment with bazedoxifene, although without changes in PINP. Coinciding with our results, Bueno et al.25 observed in a study carried out in 7,492 patients that bazedoxifene reduced bone remodeling in postmenopausal Latino women affected by osteoporosis. In this regard, it is important to note that not only the decrease in bone mineral density, but also the increase in bone remodeling is associated with an increased risk of fracture26, and that changes in osteocalcin levels after 6 months of treatment predicted the changes in bone mineral density observed after 2 years27.
Regarding bone quality, according to the parameters of the microtomography, our results showed positive effects from the treatment with bazedoxifene on the trabecular parameters BV/TV, BS/TV, Tb.Th, Tb.Sp.Tb.N. Tb.Pf, SMI, DA and Md.V and on the cortical Ct.BS/BV, although the basal values of the rats from the control group were not recovered in all cases, but they did improve compared to the ovariectomized ones.
Saito et al.28 studied ovariectomized female adult monkeys who were administered 0.2 or 0.5 mg/kg bazedoxifene for 18 months. The levels of immature and mature cross-links, BV/TV, and Tb.Th were higher in the group treated with bazedoxifene than in the ovariectomized group. However, the SMI was lower in the group treated with bazedoxifene than in the ovariectomized group. Bazedoxifene treatment prevented the deterioration of immature enzyme cross-link levels, in advanced glycosylation products, and in structural properties such as B/ TV, Tb.Th, and Tb.Pf, which significantly control the bone strength of trabecular tissue.
Regarding biomechanical parameters, we observed in our study that bazedoxifene also exerted a positive action regarding the ovariectomized rats on the maximum deformation to which the femur is subjected when performing a force on it.
Lastly, bazedoxifene produced a positive action on the weight gain experienced by rats after the ovariectomy, being a lesser weight gain than the experienced by ovariectomized rats, although higher than the experienced by the rats in the control group.
Most studies on the effects of bazedoxifene focus on vertebral fractures, such as those previously discussed14,22-24. Some authors such as Reginster et al.29 confirm that bazedoxifene also reduces non-vertebral fracture risk in women with a high risk of suffering osteoporosis. Authors such as Yavropoulou et al.5 observed an increase in lumbar BMD but not hip BMD after the treatment with bazedoxifene, but, like Reginster29, they did observe a decrease in the risk of non-vertebral fractures in high-risk postmenopausal women.
Regarding the comparative effect exerted by bazedoxifene and other drugs, in a meta-analysis carried out on 48,000 patients, Liu et al.30 observed that alendronate and risendronate produced a greater positive effect than bazedoxifene on osteoporosis, but with more side effects. Gatti et al.31 report that bazedoxifene is as effective as raloxifene in preventing bone loss in women with osteoporosis and in reducing the frequency of new vertebral fractures. Other authors such as Ellis et al.32 consider that bazedoxifene is comparable to bisphosphonates to prevent vertebral fractures among women with high-risk postmenopausal osteoporosis.
In a study carried out by our group33, we administered zoledronic acid to ovariectomized rats and we obtained much greater effects on increasing lumbar and femoral BMD on untreated rats than in the case of bazedoxifene. The rats' age conditions and ovariectomy time were totally similar to those in this study, so the results can be compared. Authors like Yavropoulou et al.5 state that bazedoxifene does not seem to offer significant advantages over other antiresorptive agents, but considering the need for long-term treatments for osteoporosis, it is a drug that has a place in the long-term therapeutic scheme to combat this sickness. Authors such as Gatti et al.31 suggest that, due to its particular profile, bazedoxifene can be considered as a second-line therapy for women between 65 and 70 years of age where bisphosphonates are contraindicated or poorly tolerated. These authors think that bazedoxifene may also be a first-place therapy in younger postmenopausal women to deal with their menopause and the prevention of osteoporosis, and that it could be prescribed alone or with conjugated estrogens.
Bibliografía
1. Riggs L, Hartmann LC. Selective estrogen receptor modulators. Mechanisms of action and application to clinical practice. N Engl J Med. 2003;348: 618-29. [ Links ]
2. Martín F, Barbancho MC. Clinical pharmacology of selective estrogen receptor modulators (SERMs). In: Cano A, Calaf J, Dueñas JL, eds. Selective estrogen receptor modulators. A new brand of multi target drugs. New York: Springer; 2006. p. 49-65. [ Links ]
3. Stump AI, Kelley KW, Wensel TM. Bazedoxifene, a third generation selective estrogen modulator for treatment of postmenopausal osteoporosis. Ann Pharmacother. 2007;41:833-9. [ Links ]
4. Pinkerton JV, Conner EA. Beyond estrogen: advances in tissue selective estrogen complexes and selective estrogen receptor modulators. Climateric. 2019; 22:140-7. [ Links ]
5. Yavropoulou MP, Makras P, Anastasilakis AD. Bazedoxifene for the treatment of osteoporosis. Expert Opin Pharmacother. 2019;20:1201-10. [ Links ]
6. McKeand W. Pharmacokinetics, dose proporcionality and bioavailability of bazedoxifene in healthy postmenopausal women. Clin Ther. 2017;39:1769-79. [ Links ]
7. Sanchez Borrego R, Lugo Salcedo F. Bazedoxifeno. Primer SERM de 3ª generación. Seguridad endometrial y en mama. Rev Osteoporos Metab Miner. 2010;2 (Suppl 5):S13-8. [ Links ]
8. Pinkerton JV. Tissue-selective estrogen complex for menopausal hormone therapy. Clin Obstet Gynecol. 2018;61:463-9. [ Links ]
9. Flores VA, Stachenfeld NS, Taylor HS. Bazedoxifene-conjugated estrogens for treating endometriosis. Obstet Gynecol. 2018;132:475-7. [ Links ]
10. Wei J, Ma L, Lai YH, Zhang R, Li H, Li C, et al. Bazedoxifene as a novel GP130 Inhibitor for colon cancer therapy. J Exp Clin Cancer Res. 2019;38(1):63. [ Links ]
11. Tian J, Chen X, Fu S, Zhang R, Pan L, Cao Y, et al. Bazedoxifene is a novel IL6/GP130 inhibitor for treating triple-negative breast cancer. Breast Cancer Res Treat. 2019;175:553-66. [ Links ]
12. Thilakasiri P, Huynh J, Poh AR, Tan CW, Nero TL, Tran K, et al. Repurposing the selective estrogen receptor modulator bazedoxifene to supress gastrointestinal cancer growth. EMBO Mol Med. 2019;11:pii e9539. [ Links ]
13. Burkhardt C, Bühler L, Tihy M, Morel P, Forni M. Bazedoxifene as a novel strategy for treatment of pancreatic and gastric adenocarcinoma. Oncotarget. 2019;10:3198-202. [ Links ]
14. Keating GM, Lyseng-Williamson KA, Duggan ST, McKeage K. Bazedoxifene: a guide to its use in postmenopausal osteoporosis. Drugs Aging. 2012;29:329-34. [ Links ]
15. Borchers RE, Gibson LJ, Burchardt H, Hayes WC. Effects of selected thermal variables on the mechanical properties of trabecular bone. Biomaterials. 1995;16:545-51. [ Links ]
16. Hahn M, Vogel M, Pompesius-Kempa M, Delling G. Trabecular bone pattern factor: A new parameter for simple quantification of bone microarchitecture. Bone. 1992;13:327-30. [ Links ]
17. Hildebrand T, Rüegsegger P. Quantification of bone microarchitecture with the structure model index. Comput Methods Biomech Biomed Eng. 1997; 1:15-23. [ Links ]
18. Hildebrand T, Rüegsegger P. A new method for the model independent assessment of thickness in three-dimensional images. J Microsc. 1997;185:67-75. [ Links ]
19. Ulrich D, van Rietbergen B, Laib A, Rüegsegger P. The ability of three-dimensional structural indices to reflect mechanical aspects of trabecular bone. Bone. 1999;25:55-60. [ Links ]
20. Ritchie O, Koester KJ, Ionova S, Yao W, Lane NE, Ager JW 3rd. Measurement of the toughness of bone: a tutorial with special reference to small animal studies. Bone. 2008;43:798-812. [ Links ]
21. Barrionuevo P, Kapoor E, Asi N, Alahdab F, Mohamed K, Benkhadra K, et al. Efficacy of pharmacological therapies for the prevention of fractures in postmenopausal women: a network metaanalysis. J Clin Endocrinol Metab. 2019; 104:1623-30. [ Links ]
22. Jin YZ, Lee JH, Xu B, Cho M. Effects of medications on prevention of secondary osteoporotic vertebral compression fracture, non-vertebral fracture, and discontinuation due to adverse events: a meta-analysis of randomized controlled trials. BMC Musculoskeletal Disord. 2019;20(1):399. [ Links ]
23. Peng L, Luo Q, Lu H. Efficacy and safety of bazedoxifene in postmenopausal women with osteoporosis. A systematic review and meta-analysis. Medicine (Baltimore). 2017;96(49):e8659. [ Links ]
24. Palacios S, Silverman SL, de Villiers TJ, Levine AB, Goemaere S, Brown JP, et al. A 7-year randomized, placebo-controlled trial assessing the long-term efficacy and safety of bazedoxifene in postmenopausal women with osteoporosis: effects on bone density and fracture. Menopause. 2015;22:806-13. [ Links ]
25. Bueno JAH, Arias L, Yu CR, Williams R, Komm BS. Efficacy and safety of bazedoxifene in postmenopausal Latino women with osteoporosis. Menopause. 2017;24:1033-9. [ Links ]
26. Garnero P, Sornay-Rendu E, Claustrat BD, Theelmas PD. Bone markers of bone turnover, endogenous hormones and the risk fractures in postmenopausal women: the OFELY study. J Bone Miner Res. 2000;15:1526-36. [ Links ]
27. Delmas PD, Hardy P, Garnero P, Daih M. Monitoring individual response to hormone replacement therapy with bone markers. Bone. 2000;26:553-60. [ Links ]
28. Saito M, Kida Y, Nishizawa T, Arakawa S, Okabe H, Seki A, et al. Effects of 18-month treatment with bazedoxifene on enzymatic immature and mature crosslinks and non-enzymatic advanced glycation end products, mineralization and trabecular microarchitecture of vertebra in ovariectomized monkeys. Bone. 2015;81:573-80. [ Links ]
29. Reginster JY, Ferrari S, Hadji P. Current challenges in the treatment of osteoporosis: an opportunity for bazedoxifene. Curr Med Res Opin. 2014;30:1165-76. [ Links ]
30. Liu GF, Wang ZQ, Liu L, Zhang BT, Miao YY, Yu SN. A network metaanalysis on the short-term efficacy and adverse events of different antiosteoporosis drugs for the treatment of postmenopausal osteoporosis. J Cell Biochem. 2018;119:4469-81. [ Links ]
31. Gatti D, Rossini M, Sblendorio I, Lello S. Pharmacokinetic evaluation of bazedoxifene for the treatment of osteoporosis. Expert Opin Drug Metab Toxicol. 2013;9:883-92. [ Links ]
32. Ellis AG, Reginster JY, Luo X, Cappelleri JC, Chines A, Sutradhar S, et al. Indirect comparison of bazedoxifene vs oral bisphosphonates for the prevention of vertebral fractures in postmenopausal osteoporotic women. Curr Med Res Opin. 2014;30:1617-26. [ Links ]
33. Martín-Fernández M, Gómez-Chinchón M, Álvarez-Galovich L, Torrubia B, Díaz-Curiel M, Guede D, et al. Effects of long-term preventive treatment with strontium ranelate and zoledronic acid on bone quality in ovariectomized rats. Am J Clin Exp Med. 2016; 4:191-200. [ Links ]
Received: December 02, 2019; Accepted: February 25, 2020











 text in
text in 


